Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
The relationship between the source and sink of atmospheric CO2 has always been a widely discussed issue in global climate change research. As one of the main overburdened parts of the earth, loess is one of the important driving factors for atmospheric CO2 consumption.
- loess
- carbonate minerals
- atmospheric CO2
- carbon sink effect
1. Introduction
The concentration of CO2 in the atmosphere increased from 295 ppmv before the industrial revolution to 417 ppmv in 2022 [1], increasing the global average temperature by 1.09 °C [2]. A key issue in global climate change research is the budget imbalance of the atmospheric carbon cycle [3,4,5,6,7]. Recent studies showed that the residual amount of the terrestrial carbon sink is 1.8~1.9 Pg C/a (1 Pg = 1015 g) [8,9], in which the carbonate weathering sink contributes to ~0.5 Pg C/a, accounting for 1/3 of missing carbon sinks (MCS) globally [10,11]. It can be calculated that there is still above 0.5 Pg of carbon that is missed in terrestrial ecosystems. Therefore, balancing the global carbon budget becomes an important task for the next stage of global carbon cycle research.
The process of carbonate weathering responds promptly to climate changes due to its rapid weathering kinetics [12,13]. It can reach reaction equilibrium within 3 h under experimental conditions [14], and its dissolution rate is ~15 times faster than that of silicate rocks. On a short timescale (less than a millennium), carbonate weathering is the main weathering carbon sink on the planet, accounting for 94% of the total rock-weathering carbon sink [10,15]. Coupled with the carbonate rock weathering, the biological carbon pumping (BCP) effect is the main contributor to long-term stable carbon sinks (a more than ten-thousand-year timescale) in the terrestrial carbon cycle [11,16]. Organic carbon transformed from the inorganic stage under BCP is stored in lakes, rivers, or oceans and then transformed into stable carbon [17,18].
On a global scale, about 6~10% of the land surface is covered by loess [19,20,21], with the largest area of loess being found in China (640,000 km2 and a thickness >250 m). Because of this, doing loess research in China has several natural advantages. The overall weight of Chinese loess is 1.89~9.47 × 104 Pg [20,22]. Carbon in loess includes the pool of organic carbon and inorganic carbon. A soil’s organic carbon and cation exchange capacity are the most important factors determining chemical deposits [23]. An inorganic carbon pool is about 850 PgC and 2~5 times higher than an organic carbon pool [24]. Due to the alkaline character of loess, recent research has demonstrated that soil with more water and a decrease in temperature can increase the abiotic fixation of CO2 [25]. Soil organic carbon (SOC) stability is a fundamental issue in understanding the evolution of the soil carbon pool, which is influenced by changes in the ecological environment [26]. In addition, studies have shown that with the development of social urbanization, agricultural carbon emissions and carbon absorption will be influenced by the adaptive alterations of farmed land’s input structure and planting structure [27]. Under continual erosion, soil carbon storage on the slopes of the Loess Plateau reached a new equilibrium state, and erosion resulted in a net sink of atmospheric CO2 on the side of degraded hillsides [28]. In addition, the inorganic carbon pool has amounted to 2~8 times the carbon emissions of fossil fuels since the industrial revolution [29]. Chinese loess has a high content of carbonate minerals (10~20%) [30,31,32,33]. According to provenance research, loess carbonate minerals are divided into primary carbonate minerals (PCM) and secondary carbonate minerals (SCM) [34]. PCM are eolian sedimentation from a faraway source area and disposed of with loess dust in a detrital form. SCM are also called pedogenic carbonates, which are generated during accumulation and soil development. Parent materials are the sedimentation of CaCO3 after the chemical weathering of primary CaCO3 and calcium-bearing silicate minerals. Its formation environment is arid to semi-humid climatic conditions with a soil pH > 7 and annual precipitation <750 mm [35]. The model is based on the calcium and carbon cycle in loess, showing the dissolution of carbonate and silicate minerals, as shown in Figure 1. During PCM dissolution, atmospheric/soil CO2 will be absorbed, and then the carbonate minerals will crystallize and deposit as SCM. In this process, the same amount of CO2 is consumed and released. Therefore, it is a carbon shift but not a carbon sink, as reported by [36]. The silicate minerals will absorb 2 mol CO2 during the dissolution process, one will be fixed in the SCM, and one will be released into the soil or atmosphere. According to current carbon cycle theory, this is a net carbon sink process [37].
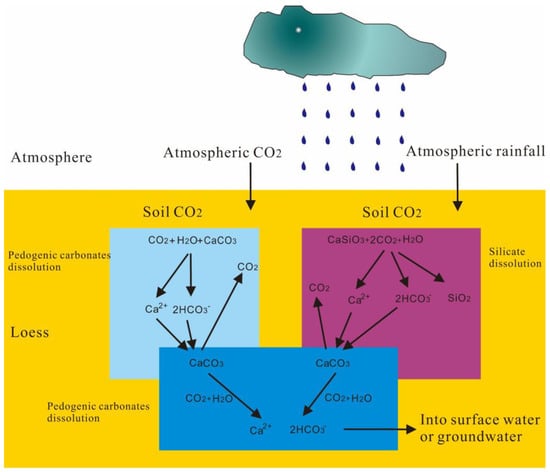
Figure 1. Schematic diagram of carbonate dissolution and carbon cycle process in loess.
2. The Influencing Factors of Loess Carbon Sink
2.1. Temporal and Spatial Distribution Characteristics of Soil CO2 in the Loess Region and Its Influencing Factors
Comprehensive environmental factors influence the loess carbon cycle. Ref [104] analyzed the hydrothermal factors on the daily and seasonal changes in soil respiration in pea farmland on the Loess Plateau by using dynamic closed gas chamber infrared CO2 analysis (IRGA). The dissolution of SCM in the soil was the main driving force for the negative flux of soil respiration (carbon sink), and low temperatures in winter aggravated it. Ref. [105] carried out research on farmland and adjacent grassland, showing that changes in hydrothermal factors caused significant differences in soil CO2 emission fluxes in these two ecosystems. The correlation between soil temperature and CO2 content was higher than soil moisture in agricultural land, fallow grassland, and jujube woodland in hilly loess regions [106]. There was a significant correlation between soil respiration CO2 and plant growth under different land-use patterns in loess [107]. Under the four planting coverings of Platycladus orientalis, Caragana korshinskii Kom., Hippophae rhamnoides Linn., and Pinus tabuliformis Carr., the relationship between soil moisture content and soil temperature of different vegetation was significantly different which resulted in the difference in soil CO2 flux [108]. Additionally, soil CO2 emission flux (take alfalfa as an example) increased with planting years [109]. CO2 levels are affected by various factors, including land management practices, plant cover, and weather conditions (rainfall, air temperature, soil temperature, soil moisture, etc.), so further research on the interaction between the above factors needs more study.
To clarify the influence of soil CO2 on the dissolution process of loess minerals, the authors of [110] calculated the amount of soil CO2 fixed during the formation of soil SCM according to the stoichiometric equilibrium. It was found that the average amount of CO2 fixed by soil at different layers was 27.9 g CO2/kg. The fixed amount of CO2 in this process is very weak because CO2 will be released again during the formation of SCM, as shown in Figure 1, and only a very small amount of DIC from SCM comes into the water as a carbon sink.
According to the balance of CO2 before and after the carbonate mineral dissolution, the authors of [111] calculated the karst CO2 uptake in a typical small loess watershed (Bahe River watershed in the loess plateau of China). Its basic principle is to subtract the free CO2 in groundwater after carbonate weathering from atmospheric precipitation, as shown in the Formula (11).
ETC = RTC − STC
Among them:
-
ETC is the amount of CO2 absorbed during carbonate weathering.
-
RTC is the total amount of CO2 in atmospheric precipitation.
-
STC is the total amount of CO2 lost in groundwater.
The study found that the pH and HCO3− content in groundwater is close to the value of the limestone area. Specifically, the carbonate mineral in loess also experienced a strong weathering process. The erosion process of the loess absorbs about 82% of the total free CO2 in the rainwater, and 18% is left in groundwater. Meanwhile, the pH value and HCO3− content in groundwater increased compared with rainwater, indicating CaCO3 dissolution. As a result, the carbon sink intensity of the Bahe loess watershed can be calculated as 2.18 t CO2/km2·a (a is the unit of the year). Thus, the CO2 sink flux in the whole loess plateau of China is 1.37 million t/a.
However, this model does not consider the soil CO2 that would be dissolved in seepage and participate in mineral erosion. Ref. [43] stated that the CO2 concentration in loess is 10 times more than in the atmosphere. The high partial pressure of CO2 in loess would promote carbonate erosion and enhance the carbon sink flux.
This shows that researchers have acknowledged the significance of loess carbonate to the global carbon cycle, conducted some related investigations, and obtained some findings. On the other hand, most of these investigations examine the soil without discussing the carbonate dissolving process and its reaction mechanism, and the related research techniques need to be improved.
2.2. Mineral Chemical Weathering Rate, CO2 Consumption, and Its Influencing Factors Watershed of the Loess Area
Researchers gathered and evaluated rock weathering rate data estimated by the Galy model and the hydro-chemical approach in a number of watersheds to better understand the factors that affect rock (mineral) weathering rate and to evaluate the extent to which weathering rates vary across the loess region (Table 2). The findings demonstrate that the weathering rate of rocks (minerals) is affected by factors including the composition of the rocks and minerals they are composed of, as well as environmental factors such as rainfall and air temperature. The rate at which silicate rocks (minerals) and carbonate rocks are weathered is positively correlated with precipitation and temperature. The correlation coefficients between rainfall and silicate rock (mineral) weathering rate and carbonate rock (mineral) weathering rate are R2 = 0.7697 and R2 = 0.8914, respectively, while the temperature is related to the silicate rock (mineral) weathering rate and carbon. The correlation coefficients of salt rock (mineral) weathering rates are R2 = 0.3776 and R2 = 0.6623, respectively (Figure 4). The chemical weathering rate has a strong positive relationship with precipitation, as shown by the fact that it increases with both rainfall and temperature [112]; this is in agreement with the findings that the influence of rainfall was greater than that of temperature. It is consistent with the correlations, and it is evident that rainfall and temperature have different effects on the weathering rates of silicate rocks (minerals) and carbonate rocks (minerals) in contrast to carbonate rocks (minerals). It is more influenced by rainfall and temperature and is more sensitive to weathering than silicate rocks (minerals), suggesting the effect of the properties of the rocks and minerals themselves on the rate of weathering of rocks (minerals) in the catchment. In summary, the chemical weathering rate and carbon sink capacity of a watershed depend not only on the lithological features of the watershed but also on the rainfall and temperature of the watershed. The loess area (Qingliangsi River) is relative to the average annual rainfall of the Nenjiang River and Songhua River, so it has the same order of magnitude of rock (mineral) weathering rate, while the Yangtze River, Wujiang, and other basins have higher annual average rainfall and annual average temperature than the Qingliangsi River; therefore, the rock chemical weathering rate and CO2 consumption rate in the study area are much lower than those of these watersheds. Although the total amount of ions in the Qingliangsi River water body is significantly higher than the global average ion content, the weathering rate of its loess minerals is lower than the average chemical weathering rate of the global rocks. The primary causes may have two points: silicate minerals account for the vast majority. As stated above, silicate minerals have strong weathering opposition, and their weathering rate is much lower than that of carbonate minerals; on the other hand, Qingliangsi is located in the Loess Plateau, where evaporation is strong, and the annual average evaporation amount is 4.9 times the average annual rainfall, which is the primary cause of the weak chemical weathering in the Qingliangsi watershed. Nevertheless, regarding the spatial heterogeneity of rock weathering rates, more research sites are needed to see comparable work, particularly in the loess area.
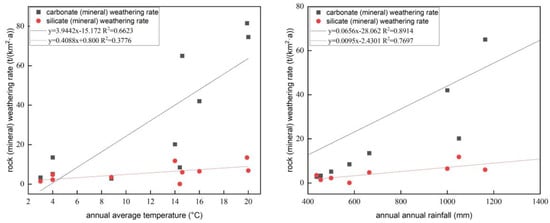
Figure 4. Relationship between rock (mineral) weathering rate, rainfall, and temperature.
Table 2. CO2 consumption rate and flux of mineral weathering.
| Watershed | Annual Average Temperature | Annual Annual Rainfall | Carbonate (Mineral) Weathering Rate | Silicate (Mineral) Weathering Rate | Rock (Mineral) Weathering Rate | CO2 Consumption Rate | Reference |
|---|---|---|---|---|---|---|---|
| °C | mm | t/(km2·a) | t/(km2·a) | t/(km2·a) | 103 mol/(km2·a) | ||
| Qingliangsi River (Loess area) | 8.8 | 437.3 | 2.83 | 3.49 | 9.31 | 144.1 | [54] |
| Sanchuan River | 9.2 | 467.7 | — | — | 7.84 | 120 | unpublished data |
| Yellow River | — | — | 9.92 | 2.02 | 36.46 | 169 | [113] |
| Yangtze River | — | — | 55.86 | 5.25 | 64.99 | 611 | [59] |
| Songhua River | 4 | 500 | 5.15 | 2.23 | 7.38 | 120 | [114] |
| Second Songhua River | 4 | 664 | 13.50 | 4.74 | 18.24 | 268 | [114] |
| Nenjiang River | 3 | 455 | 3.31 | 1.39 | 4.70 | 75 | [115] |
| Pearl River | 20 | 1000~2000 | 74.53 | 6.87 | — | 620.36 | [116] |
| Wujiang River | 14.6 | 1163 | 65 | 6 | 108.5 | 902 | [114] |
| Yalong River | 16 | 1000 | 42.0 | 6.5 | — | 281 | [117] |
| Qingshui River | 14 | 1050 | 20.16 | 11.77 | 109.97 | 725 | [118] |
| Bishuiyan River | 19.9 | 1685.5 | 81.51 | 13.46 | 93.10 | 853.02 | [119] |
| Qin River | 14.4 | 578.5 | 8.47 | 0.07 | 16.92 | 146 | [120] |
| Amazon River | — | — | 11.08 | 13.04 | 49.15 | 157 | [89] |
| Global Median | — | — | — | — | 36 | 246 | [59] |
This entry is adapted from the peer-reviewed paper 10.3390/land12010133
This entry is offline, you can click here to edit this entry!
