Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Melanoma, a neoplasm arising from malignant transformation of melanocytes, is the most lethal form of skin cancer.
- oxidative stress
- immune response
- cancer
- melanoma
1. Melanoma Pathophysiology and Current Options for Treatment
Melanoma, a neoplasm arising from malignant transformation of melanocytes, is the most lethal form of skin cancer [1]. However, melanoma can also develop on mucosal surfaces such as the oral cavity, the genital mucosa, the upper gastrointestinal mucosa as well as the uveal tract of the eye and leptomeninges [2]. The incidence of cutaneous melanoma has rapidly increased over the past decades. Melanoma is the ninth most common malignancy and the second for mortality, with an incidence being markedly increased in patients with a history of heavy sun exposure or isolated episodes of serious sunburn [3][4]. Although the majority of primary melanomas are cured with local wide excision, metastatic melanoma carries a grim prognosis, with a median survival of nine months and a long-term survival rate of 10% [5]. Cancer metastasis is considered the end stage of the progression of any tumour. It is composed of different steps that include infiltration of cancerous cells into the neighboring tissue, followed by intravasation as tumour cells undergo trans-endothelial migration through the vessel wall and, finally, extravasation and proliferation at the distant organ to form secondary tumours [6][7]. About half of all melanomas carry mutations in the BRAF gene, which makes these tumours amenable to targeted therapy. BRAF V600 mutation-positive unresectable or metastatic melanoma in adults is treated with the selective competitive inhibitor of BRAF kinase dabrafenib as monotherapy or in combination with the MEK inhibitor trametinib. Other treatment options are represented by the immune checkpoint inhibitors, which include the PD-1 inhibitors nivolumab and pembrolizumab and the CTLA-4 inhibitor ipilimumab. Second-line therapy is achieved by chemotherapy with alkylating cytostatic dacarbazine (DTIC), amongst others. Radiation therapy can be a useful treatment in some clinical settings including adjuvant therapy after complete excision of a primary melanoma or after therapeutic lymphadenectomy [8][9].
2. Oxidative Stress and Melanocytes
A distinctive feature of melanoma compared to other solid tumours is the especially high oxidative stress level, which can be explained by both extrinsic and intrinsic factors [2][10][11]. Due to their physical location, melanocytes are directly exposed to environmental factors inducing oxidative stress, such as UV radiation [12][13]. Epidemiological studies have demonstrated a strong association between UV radiation and melanoma risk. UV light is a type of electromagnetic radiation emitted by the sun. The UV spectrum is conventionally subdivided into UVA radiation (320–400 nm), UVB radiation (280–320 nm), and UVC radiation (100–280 nm). Only UVA radiation and a portion of the UVB spectrum (above approximately 300 nm) can reach the surface of the earth. Thus, both UVA and UVB may contribute to melanoma development [14]. UV radiation can lead to indirect oxidation-mediated damage of cutaneous macromolecules by stimulating reactive oxygen species production through enzymatic reactions catalyzed by enzymes such as NADPH oxidase (NOX1 and NOX4), cyclo-oxygenase, and xanthine oxidase, or by the damage of mitochondrial respiratory chain enzymes. Alternatively, UV induces the skin to also produce high levels of reactive nitrogen species (NO and possibly ONOO−) [15]. When UV-stimulated reactive oxygen species target DNA molecules, various types of oxidative DNA lesions are induced, including DNA single-strand breaks, DNA–protein crosslinks, and alteration of DNA nitrogenous bases. In particular, the oxidation of the guanine bases, which produces 8-oxo-7,8-dihydroguanine (8-oxoG), is the most abundant form of oxidative DNA damage [16] (Figure 1). These alterations can induce inflammation and can further initiate tumorigenesis [17]. Reactive species and damaged DNA can activate intracellular protein complexes such as inflammasomes [18][19]. In this context, both keratinocytes and melanocytes secrete cytokines with pro-inflammatory action, thus modulating innate and adaptive immune responses [20]. All the immune-related molecules, cytokines, chemokines, and non-immune molecules, such as growth factors have both paracrine and autocrine effects upon the microenvironment and design the local milieu that initiates and then regulates local inflammation or can lose control, consequently favouring the process of tumorigenesis. Inflammation has acute and chronic stages, but its link to tumorigenesis is carried out by chronic inflammation [21]. During inflammatory response, mast cells and monocytes/macrophages are recruited [22]. In particular, mast cells are the first to migrate to the site of proliferation; macrophages follow later in the response. Both are capable of producing reactive species as a cytotoxic mediator to kill cells [23]. Reactive oxygen species can react with the nucleic acids attacking the nitrogenous bases and the sugar phosphate backbone and can evoke single- and double-stranded DNA breaks [24]. While acute inflammation is regulated by T-helper (Th)1-polarized T lymphocytes attracted by innate immune cells, secreting mainly anti-tumour immune molecules such as interleukin (IL)-2 and interferon (IFN)-γ, chronic inflammation is controlled by regulatory T cells (Tregs), Th2 cells, that secrete pro-tumorigenic factors (IL-4, IL-6, IL-10, IL-13 and transforming growth factor (TGF)-β) [25]. In this regard, reactive species produced by melanoma cells and tumor-infiltrating leukocytes, including Tregs, can suppress immune responses [24].
Additionally, UV-induced reactive oxygen species also attack other major biomolecules, causing protein oxidation and lipoperoxidation that compromise cellular ultrastructure and function [26]. In fact, when UV radiation hits the skin, within sebaceous lipids, squalene is oxidized and can initiate inflammatory processes, thus acting as an inflammasome activation danger signal (Figure 1). In this regard, it is worth mentioning that melanocytes are more vulnerable to UV-mediated oxidative injury than other skin cells, such as keratinocytes and fibroblasts, since their specialized function, namely the melanin synthesis, is an energy-consuming process that itself contributes to generating a large amount of reactive oxygen species [11]. In fact, there are conflicting data in the literature on the pro-oxidant and antioxidant effects exerted by melanin [27]. The presence of melanin in the skin appears to be a double-edged sword: it protects melanocytes through its capacity to absorb UV radiation, but its synthesis in melanocytes results in higher levels of intracellular reactive oxygen species that may increase melanoma susceptibility. During the melanin biosynthesis, tyrosinase enzyme oxidizes tyrosine to L-DOPA, which itself is oxidized to DOPA-quinone, a reactive molecule toward thiols and/or amino groups. Afterward, a redox exchange converts the DOPA-quinone into DOPA-chrome, which, after a decarboxylation yields dihydroxy-indole, or alternatively, after tautomerization produces dihydroxy-indole carboxylic acid. The process that converts indoles to quinones implicates an important generation of reactive oxygen species (O2•− and H2O2) (Figure 1) [28][29]. Finally, the polymerization of the quinones results in the formation of black-brown eumelanin. Instead, the pheomelanin, which displays a typical red-yellow colour, differs from the eumelanin for having a higher ratio of sulphur to quinones, and its biogenesis process has as intermediate the generation of cysteinyl-DOPA instead of L-DOPA. These variations are responsible for the higher pro-oxidant effects caused by the sunlight of pheomelanin with respect to eumelanin. Eumelanin is a good free radical scavenger; pheomelanin is not, and its benzothiazole units can act as photosensitizers leading to the production of reactive oxygen species [25][29][30][31][32]. Paradoxically, while high levels of reactive oxygen species can cause oxidative stress and induce cell death, low levels of superoxide and H2O2 can promote G1→S cell cycle transition. Thus, oxidative stress or redox status shifts may cause cell transition from a quiescent to a proliferative status, growth arrest, or cell death, according to the duration and extent of the redox imbalance [33].
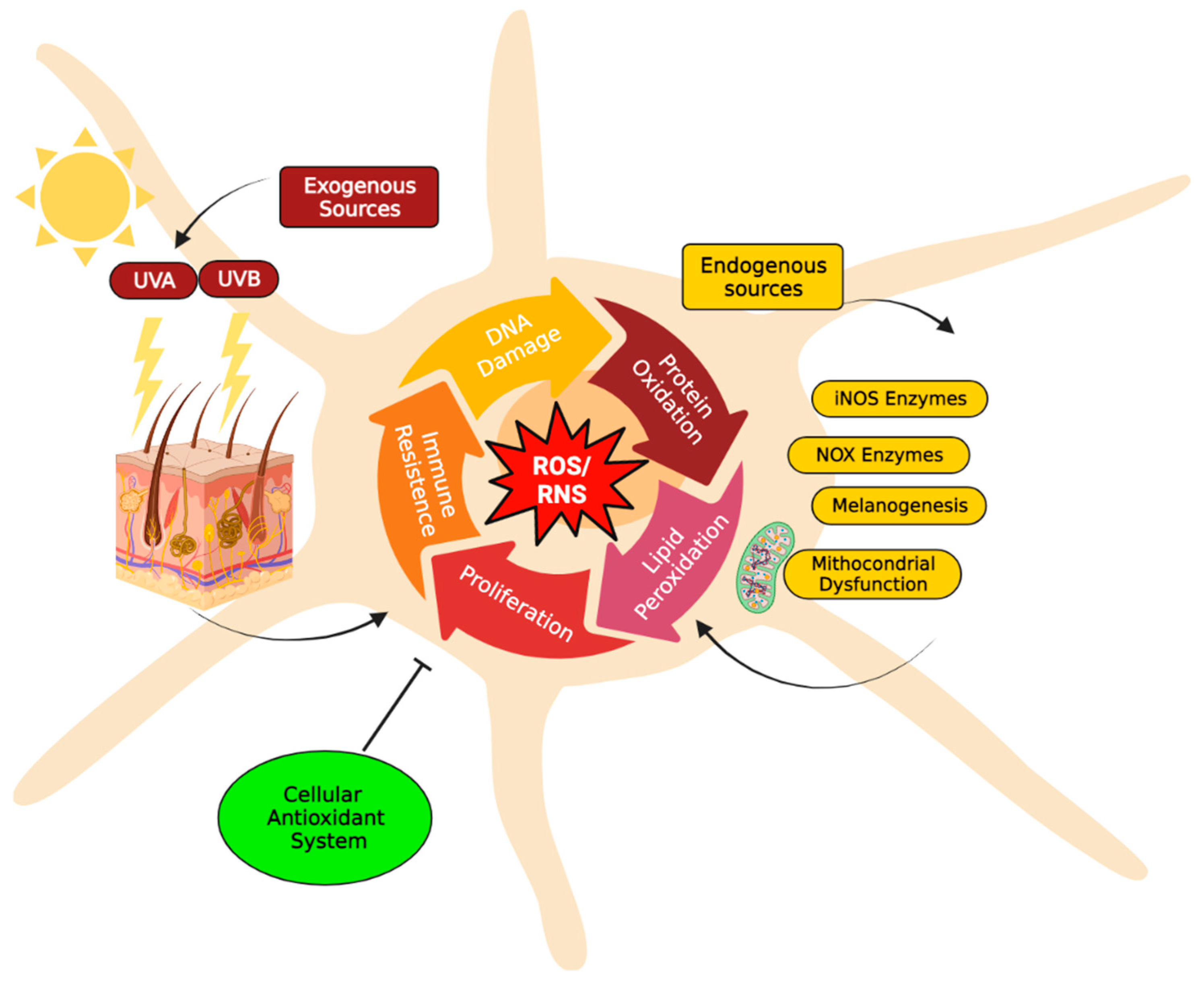
Figure 1. Major reactive species sources in melanocytes. The increase of reactive oxygen species (H2O2, O2−•) and/or reactive nitrogenous species (NO and ONOO−) induces severe damages to major biomolecules, resulting in DNA and protein oxidation, as well as lipoperoxidation, that compromise cellular structure and function. Consequently, these alterations can induce inflammation and can initiate tumorigenesis processes (e.g., cell proliferation and adaptive immune resistance). To maintain acceptable levels of reactive species, melanocytes cells usually increase their antioxidant systems to protect cells from oxidative stress damage and restore physiological redox balance. The redox balance in the cell is normally regulated by a complex antioxidant system. Endogenous antioxidants include catalase (CAT), superoxide dismutase (SOD), glutathione peroxidases (GPXs) and glutathione (GSH). In particular, GSH metabolism protects melanocytes from the toxic effects of H2O2 formed during melanin synthesis. GSH metabolism, therefore, appears to be critically important to the maintenance of melanocyte cell viability [34]. Instead, natural antioxidant compounds can be obtained from the diet, e.g., beta-carotene (vitamin A), alpha-ascorbic acid (vitamin C), tocopherol (vitamin E) [15]. The figure was created using BioRender.com.
3. Melanocytes and Immune Response
Accumulating evidence supports the concept that melanocytes are not only professional melanin-producing cells but are also active factors in the cutaneous immune system [35][36]. The production of melanin involves stepwise oxidation of the amino acid tyrosine and downstream aromatic compounds. Myelinization has important protective roles in several species, as toxic intermediates (semi-quinone, DOPA-quinone and indole-quinone) may be produced, including reactive oxygen species. These intermediate compounds are believed to exert strong antimicrobial activities, and melanin, the end-product of myelinization, may have the capacity to trap, inhibit, and even kill invading bacteria and other microorganisms [37][38].
Melanin may also have a crucial immune-regulatory role. It has been found to have immune-modulatory activities through inhibition of pro-inflammatory cytokine production by T lymphocytes, monocytes, fibroblasts, and endothelial cells. The transfer of acidified melanin-containing organelles (melanosomes) from melanocytes to neighboring keratinocytes in the outer portions of the epidermis may have a role in acidifying the stratum corneum in darkly pigmented skin [39]. Acidity in the stratum corneum could enhance skin barrier function and the integrity and/or cohesion of stratum corneum; it might also exert antimicrobial function [40]. In response to different stimuli, melanocytes could also regulate cutaneous immune response by producing and releasing several immune-suppressive molecules, e.g., alpha-melanocyte stimulating hormone (a-MSH). The latter participates in both anti-inflammatory and immunomodulatory activities [41]. In this context, it has also been demonstrated that melanocytes are capable of phagocytosis. In this regard, melanosomes have functional and structural similarities to lysosomes, and have been considered as indeed specialized lysosomes. Because phagocytosis is understood to be a prerequisite for antigen processing and presentation, phagocytosis by melanocytes suggests that the melanocytes have antigen presentation potential [35]. Finally, human melanocytes express functional toll-like receptors (TLRs). Upon ligation of TLRs with lipopolysaccharide, these cells may trigger NF-kB and/or mitogen-activated protein kinase signaling pathways, thus producing several pro-inflammatory cytokines and chemokines. These molecules may modulate the recruitment and activation of different immune cells in the skin. Thus, the expression of functional TLRs on melanocytes suggests that they may act as early sensors in immune responsiveness [42].
4. Immune Evasion in Melanoma and Potential Novel Options for treatment
Melanoma is also an immunologic malignancy [43]. These cancer cells are constantly adapting to the host defenses by manipulating intrinsic and extrinsic biological pathways [43]. In the event of the onset and development of melanoma, the immune system is exposed to numerous previously unseen antigens that are derived from genetic abnormalities. In this context, the immune system can recognize and eliminate some cancers at an early stage of their development. The adaptive immune system appears to be of fundamental importance in the antitumor response, which is triggered by activation of a wide range of diverse and highly specific receptors on T and B cells. An effective immune response begins when the T or B cells recognize the tumor antigen in a pro-stimulatory context and undergo activation and proliferation. B cells have as a receptor a surface IgM immunoglobulin and are able to recognize soluble antigens, bind to them and differentiate into plasmacytes, which secrete large amounts of highly specific antibodies [44]. Unfortunately, melanoma cells may develop numerous immuno-evasive mechanisms that allow them to resist natural or therapy-induced immune attacks [45]. Through these mechanisms, tumour cells are capable of modulating themselves and their surroundings in order to promote their survival, growth, and invasion, even under persistent immune pressure. Indeed, stressors present in the tumour microenvironment, such as chronic hypoxia, play crucial roles in promoting tumour cell plasticity and heterogeneity, which finally leads to the acquisition of immune tolerance and tumour progression [46][47]. The plasticity of melanoma cells leads to a phenomenon called immune escape, whereby cancer cells acquire a less immunogenic phenotype and the ability to suppress anti-tumour immune cells within the tumour microenvironment [21][48][49]. Although the introduction of the immune checkpoint inhibitors mentioned above has undoubtedly represented a great advancement in the treatment of melanoma and has improved patient prognosis, many patients do not respond to therapy and consequently remain with limited options for treatment. Novel treatment options might include newer checkpoint inhibitors such as B- and T-lymphocyte attenuator (BTLA), lymphocyte- activation gene 3 (LAG-3), and T-cell immunoglobulin and mucin domain-3 (TIM-3) inhibitors [50]. These are subject of intense investigation in preclinical and clinical studies [51][52][53].
A possible alternative strategy to improve therapeutic efficacy can be targeting the redox balance in cancer cells [54]. Ion channels are transmembrane proteins that connect the inside of the cell to its outside in a selective fashion by regulating the ionic permeability of cell membranes. Ion channels represent an important class of biomolecules due to their ability to serve as key elements in signaling and sensing pathways [55][56][57][58]. Over the past 10 years, it became obvious that ion channels play a key role in cancer development by influencing cell migration, cell cycle progression, and proliferation [59][60][61][62][63]. During the transition from a normal cell towards a cancer cell, a series of genetic alterations occur, which may also affect ion channel expression, or may cause a change in ion channel activity. To name just some examples, cell migration is important not only for initiation of metastasis [64], but also plays a critical role for the homing of tumour-infiltrating lymphocytes [65]. In addition, ionic (calcium) signalling might influence the tumour microenvironment and change the fate of the melanoma by altering the function of innate and adaptive immune cells and regulating extracellular matrix and tumour vascularization, thus adapting to different physical and chemical surroundings [66]. Since ion channels are mostly localized to the plasma membrane, they can be subjected to multiple layers of regulation, and therefore represent promising targets for therapeutic intervention in cancer. Indeed, reactive oxygen species production can directly induce post-translational modification of ion channels leading to oxidation and/or nitration of specific amino acid residues or indirectly modulate channel function by affecting the intracellular signaling pathways [67].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24010887
References
- Ostrowski, S.M.; Fisher, D.E. Biology of Melanoma. Hematol. Clin. N. Am. 2020, 35, 29–56.
- Kibbi, N.; Kluger, H.; Choi, J.N. Melanoma: Clinical Presentations. Melanoma 2015, 167, 107–129.
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495.
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254.
- Turner, N.; Ware, O.; Bosenberg, M. Genetics of metastasis: Melanoma and other cancers. Clin. Exp. Metastasis 2018, 35, 379–391.
- Yang, S. The store-operated calcium channels in cancer metastasis from cell migration invasion to metastatic colonization. Front. Biosci. 2018, 23, 1241–1256.
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6.
- American Cancer Society. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/treating.html (accessed on 28 November 2022).
- Radiation Therapy in the Management of Melanoma. Available online: www.uptodate.com/contents/radiation-therapy-in-the-management-of-melanoma) (accessed on 28 November 2022).
- Venza, M.; Visalli, M.; Beninati, C.; De Gaetano, G.V.; Teti, D.; Venza, I. Cellular Mechanisms of Oxidative Stress and Action in Melanoma. Oxidative Med. Cell. Longev. 2015, 2015, 481782.
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Contemp. Oncol./Współczesna Onkol. 2022, 25, 1–7.
- Sample, A.; He, Y.-Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2017, 34, 13–24.
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23.
- Jin, S.-G.; Padron, F.; Pfeifer, G.P. UVA Radiation, DNA Damage, and Melanoma. ACS Omega 2022, 7, 32936–32948.
- Pizzimenti, S.; Ribero, S.; Cucci, M.A.; Grattarola, M.; Monge, C.; Dianzani, C.; Barrera, G.; Muzio, G. Oxidative Stress-Related Mechanisms in Melanoma and in the Acquired Resistance to Targeted Therapies. Antioxidants 2021, 10, 1942.
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124.
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The Role of Inflammation in Skin Cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469.
- Schneider, S.L.; Ross, A.L.; Grichnik, J.M. Do inflammatory pathways drive melanomagenesis? Exp. Dermatol. 2014, 24, 86–90.
- Wittgen, H.G.M.; Van Kempen, L.C.L.T. Reactive oxygen species in melanoma and its therapeutic implications. Melanoma Res. 2007, 17, 400–409.
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis (Review). Oncol. Lett. 2018, 17, 4068–4084.
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41.
- van Kempen, L.C.; van Kempen, L.C.L.; van Muijen, G.N.P.; Ruiter, D.J. Stromal responses in human primary melanoma of the skin. Front. Biosci. 2005, 10, 2922–2931.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxid. Med. Cell. Longev. 2016, 2016, 1580967.
- Liu-Smith, F.; Poe, C.; Farmer, P.J.; Meyskens, F.L. Amyloids, melanins and oxidative stress in melanomagenesis. Exp. Dermatol. 2014, 24, 171–174.
- Pecorelli, A.; Valacchi, G. Oxidative-Stress-Sensitive microRNAs in UV-Promoted Development of Melanoma. Cancers 2022, 14, 3224.
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537.
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200.
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment. Cell Melanoma Res. 2009, 22, 563–579.
- Napolitano, A.; Panzella, L.; Monfrecola, G.; D’Ischia, M. Pheomelanin-induced oxidative stress: Bright and dark chemistry bridging red hair phenotype and melanoma. Pigment. Cell Melanoma Res. 2014, 27, 721–733.
- Panzella, L.; Leone, L.; Greco, G.; Vitiello, G.; D’Errico, G.; Napolitano, A.; D’Ischia, M. Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment. Cell Melanoma Res. 2014, 27, 244–252.
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612.
- Obrador, E.; Liu-Smith, F.; Dellinger, R.W.; Salvador, R.; Meyskens, F.L.; Estrela, J.M. Oxidative stress and antioxidants in the pathophysiology of malignant melanoma. Biol. Chem. 2018, 400, 589–612.
- Meyskens, F.L., Jr.; Farmer, P.; Fruehauf, J.P. Redox Regulation in Human Melanocytes and Melanoma. Pigment Cell Res. 2001, 14, 148–154.
- Hong, Y.; Song, B.; Chen, H.-D.; Gao, X.-H. Melanocytes and Skin Immunity. J. Investig. Dermatol. Symp. Proc. 2015, 17, 37–39.
- Speeckaert, R.; van Geel, N.; Vermaelen, K.V.; Lambert, J.; Van Gele, M.; Speeckaert, M.M.; Brochez, L. Immune reactions in benign and malignant melanocytic lesions: Lessons for immunotherapy. Pigment. Cell Melanoma Res. 2010, 24, 334–344.
- Burkhart, C.G.; Burkhart, C.N. The mole theory: Primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int. J. Dermatol. 2005, 44, 340–342.
- Gasque, P.; Jaffar-Bandjee, M.C. The immunology and inflammatory responses of human melanocytes in infectious diseases. J. Infect. 2015, 71, 413–421.
- Mohagheghpour, N.; Waleh, N.; Garger, S.J.; Dousman, L.; Grill, L.K.; Tusé, D. Synthetic Melanin Suppresses Production of Proinflammatory Cytokines. Cell. Immunol. 2000, 199, 25–36.
- Gunathilake, R.; Schurer, N.Y.; Shoo, B.A.; Celli, A.; Hachem, J.-P.; Crumrine, D.; Sirimanna, G.; Feingold, K.R.; Mauro, T.M.; Elias, P.M. pH-Regulated Mechanisms Account for Pigment-Type Differences in Epidermal Barrier Function. J. Investig. Dermatol. 2009, 129, 1719–1729.
- Luger, T.A.; Brzoska, T.; Scholzen, T.E.; Kalden, D.-H.; Sunderkötter, C.; Armstrong, C.; Ansel, J. The Role of α-MSH as a Modulator of Cutaneous Inflammation. Ann. N. Y. Acad. Sci. 2006, 917, 232–238.
- Plonka, P.M.; Passeron, T.; Brenner, M.; Tobin, D.J.; Shibahara, S.; Thomas, A.; Slominski, A.; Kadekaro, A.L.; Hershkovitz, D.; Peters, E.; et al. What are melanocytes really doing all day long…? Exp. Dermatol. 2009, 18, 799–819.
- Eddy, K.; Chen, S. Overcoming Immune Evasion in Melanoma. Int. J. Mol. Sci. 2020, 21, 8984.
- Giavina-Bianchi, M.H.; Junior, P.F.G.-B.; Neto, C.F. Melanoma: Tumor microenvironment and new treatments. An. Bras. de Dermatol. 2017, 92, 156–166.
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080.
- Moogk, D.; Da Silva, I.P.; Ma, M.W.; Friedman, E.B.; De Miera, E.V.-S.; Darvishian, F.; Scanlon, P.; Perez-Garcia, A.; Pavlick, A.C.; Bhardwaj, N.; et al. Melanoma expression of matrix metalloproteinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy. J. Transl. Med. 2014, 12, 342.
- Armani, G.; Pozzi, E.; Pagani, A.; Porta, C.; Rizzo, M.; Cicognini, D.; Rovati, B.; Moccia, F.; Pedrazzoli, P.; Ferraris, E. The heterogeneity of cancer endothelium: The relevance of angiogenesis and endothelial progenitor cells in cancer microenvironment. Microvasc. Res. 2021, 138, 104189.
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359.
- Pansy, K.; Uhl, B.; Krstic, J.; Szmyra, M.; Fechter, K.; Santiso, A.; Thüminger, L.; Greinix, H.; Kargl, J.; Prochazka, K.; et al. Immune Regulatory Processes of the Tumor Microenvironment under Malignant Conditions. Int. J. Mol. Sci. 2021, 22, 13311.
- Marzagalli, M.; Ebelt, N.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250.
- Dong, X.; Song, J.; Chen, B.; Qi, Y.; Jiang, W.; Li, H.; Zheng, D.; Wang, Y.; Zhang, X.; Liu, H. Exploration of the Prognostic and Immunotherapeutic Value of B and T Lymphocyte Attenuator in Skin Cutaneous Melanoma. Front. Oncol. 2021, 10, 592811.
- Zhao, L.; Wang, H.; Xu, K.; Liu, X.; He, Y. Update on lymphocyte-activation gene 3 (LAG-3) in cancers: From biological properties to clinical applications. Chin. Med. J. 2022, 135, 1203–1212.
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911.
- Trzeciak, E.R.; Zimmer, N.; Gehringer, I.; Stein, L.; Graefen, B.; Schupp, J.; Stephan, A.; Rietz, S.; Prantner, M.; Tuettenberg, A. Oxidative Stress Differentially Influences the Survival and Metabolism of Cells in the Melanoma Microenvironment. Cells 2022, 11, 930.
- Jentsch, T.J.; Hübner, C.A.; Fuhrmann, J.C. Ion channels: Function unravelled by dysfunction. Nat. Cell Biol. 2004, 6, 1039–1047.
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 solute carriers during oxidative stress. Acta Physiol. 2022, 235, e13796.
- Schmidpeter, P.A.; Nimigean, C.M. Correlating ion channel structure and function. Methods Enzymol. 2021, 652, 3–30.
- Garavaglia, M.; Dopinto, S.; Ritter, M.; Fürst, J.; Saino, S.; Guizzardi, F.; Jakab, M.; Bazzini, C.; Vezzoli, V.; Dossena, S.; et al. Membrane Thickness Changes Ion-Selectivity of Channel-Proteins. Cell. Physiol. Biochem. 2004, 14, 231–240.
- Lang, F.; Stournaras, C. Ion channels in cancer: Future perspectives and clinical potential. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130108.
- Bertelli, S.; Remigante, A.; Zuccolini, P.; Barbieri, R.; Ferrera, L.; Picco, C.; Gavazzo, P.; Pusch, M. Mechanisms of Activation of LRRC8 Volume Regulated Anion Channels. Cell. Physiol. Biochem. 2021, 55, 41–56.
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621.
- Cuddapah, V.A.; Sontheimer, H. Ion channels and tranporters in cancer. Ion channels and the control of cancer cell migration. Am. J. Physiol. Physiol. 2011, 301, C541–C549.
- Remigante, A.; Gavazzo, P.; Morabito, R.; Dossena, S. Editorial: Ion transporters and channels in cellular pathophysiology. Front. Cell Dev. Biol. 2022, 10, 1049433.
- Gambade, A.; Zreika, S.; Guéguinou, M.; Chourpa, I.; Fromont, G.; Bouchet, A.M.; Burlaud-Gaillard, J.; Potier-Cartereau, M.; Roger, S.; Aucagne, V.; et al. Activation of TRPV2 and BKCa channels by the LL-37 enantiomers stimulates calcium entry and migration of cancer cells. Oncotarget 2016, 7, 23785–23800.
- Sackstein, R.; Schatton, T.; Barthel, S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Investig. 2017, 97, 669–697.
- Zhang, H.; Chen, Z.; Zhang, A.; Gupte, A.A.; Hamilton, D.J. The Role of Calcium Signaling in Melanoma. Int. J. Mol. Sci. 2022, 23, 1010.
- Annunziato, L.; Pannaccione, A.; Cataldi, M.; Secondo, A.; Castaldo, P.; Di Renzo, G.; Taglialatela, M. Modulation of ion channels by reactive oxygen and nitrogen species: A pathophysiological role in brain aging? Neurobiol. Aging 2002, 23, 819–834.
This entry is offline, you can click here to edit this entry!
