2. Methods of Self-Healing of Polymeric Materials
Thermoplastic polymers are able to heal when the surfaces of cracks connect, and the polymer chains are entangled again [
22]. One of the simple ways to heal cracked or scratched coatings is to apply solvents or heat to the damaged area, which allows for the surface to be wetted and healed, as well as moving and entangling the polymer chains [
23]. The healing of weakly crosslinked meshes also occurs at a temperature 10–20° above their glass transition temperature (T
g). This was demonstrated in the work of [
24], where epoxy coatings were prepared by curing diglycidyl ether of bisphenol A (DGEBA) with a mixture of diamine and monoamine in the presence of 0.05–1 phr of carbon black as a photothermal filler. It was shown that an increase in the content of the latter or in the intensity of IR irradiation reduced the healing time.
This approach is unsuitable for thermoplastics and complex multicomponent composites. An analysis of the functional and structural properties of self-healing network polymers [
25,
26,
27] led to the conclusion that their development is reduced to two strategies [
8,
11,
28]: (a) using the reversibility of crosslinks [
10,
29,
30] and (b) introducing a healing agent into cracks [
12,
31,
32,
33]. In the latter case, a specific method of vascular self-healing is distinguished [
34,
35,
36,
37,
38]. In addition, polymer spacers are used in composites [
39,
40,
41].
The first (intrinsic) approach assumes that self-healing occurs due to the chemical bonds of the polymer material itself, which can heal the structure after damage under the influence of external factors such as heat, ultraviolet light, or chemicals. In other words, self-healing networks heal due to their inherent (intrinsic) properties, namely, crosslinks can migrate along the polymer chains between different positions without the risk of structural damage or the loss of material properties.
Polymers containing such dynamic bonds are known as covalent adaptable networks (CANs). Bond exchange reactions in network polymers proceed according to one of two mechanisms [
42,
43]: a dissociative process, in which crosslinks are split into their constituent reactive fragments with subsequent regeneration [
44]; or an associative process, in which a pendant reactive group enters into substitution reactions with an existing crosslink. These mechanisms are realized as interchain exchange reactions [
45], in particular, transesterification [
46,
47] and metathesis [
48,
49,
50]. In any case, the topological structure in CAN is restructured, which is triggered by pressure and temperature and leads to the healing of damages (
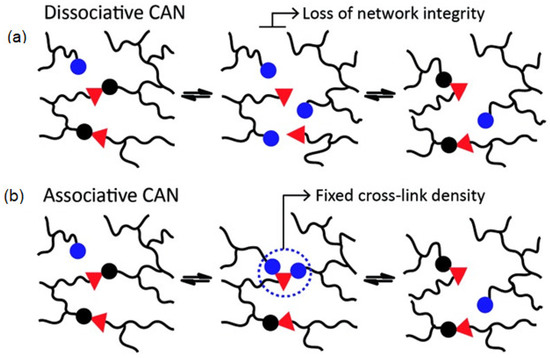
Figure 1).
Figure 1. Dissociative (
a) and associative (
b) CANs based on exchange reactions and occurring, respectively, with or without a temporary loss of crosslink density. Reproduced from [
43]. with permission from the Royal Society of Chemistry.
Associative CANs belong to the family of dynamic polymers [
51] called dynamers [
52,
53], a term introduced by J.-M. Lehn for polymers, which also includes supramolecular polymers. The dynamic properties of the latter are achieved through intermolecular interactions such as hydrogen bonds, metal–ligand (M–L) coordination, and π–π interactions [
39,
41,
54,
55]. The exchange of non-covalent bonds is also used as a dissociative method for solving problems in the self-healing of polymeric materials [
51,
56].
The second (extrinsic) method consists of the release of healing agents from containers (hollow glass fibers, capsules, microvessels), which break under the action of a crack propagating inside the material and lead to its healing [
32,
33]. Most of the healing action takes place at room temperature, but sometimes heating is required to improve healing efficiency. Efficiency is measured quantitatively as the ratio of any property of the healed material to the original, expressed as a percentage.
As a rule, the coating is more difficult to heal with the intrinsic method than its bulk counterpart. This fact is explained by the fact that the small thickness of the coating and strong adhesive bond with the substrate limit the mobility of polymer networks, which is necessary to eliminate the damage [
37]. Basically, polymer coatings are considered [
37,
56,
57] as autonomous systems that use the external healing method capable of restoring their bulk integrity or functional properties without any external physical intervention. However, the use of containers in thin layers of coatings is limited by the size of the inclusions. Thus, in the work of [
20], nanocapsules with a diameter of 100–800 nm were used.
Some systems use nanoparticles (NPs) that spontaneously migrate into cracks formed because of polymer damage [
22,
58,
59,
60]. If CdS NPs are small (3 nm) compared to the radius of gyration (R
g) of the polymer, the constraints on the chain configuration are small, and therefore the entropy cost (Δ
S) for incorporating NPs into the polymer matrix is low. However, with larger (5.2 nm) particles (comparable to R
g), Δ
S increases, due to which NPs will be more easily pushed out of the matrix into an open crack [
61].
Self-healing materials can be repaired by intrinsic methods only after crack closure, that is, their recovery consists of two stages: crack closure and healing. Therefore, the mechanisms of closure and the chemical process of the restoration of polymer structures should be especially considered [
62]. It should be noted that self-healing is different from self-adhesion. In the first case, the contacting surfaces are not in equilibrium with respect to the reactive groups, and the second case, the process is in equilibrium. Extrinsic methods require considering the relationship between the choice of the location of functional containers and the localization of stress in the composite [
63].
The introduction of thermoplastic polymers, such as copolymers of ethylene with methyl acrylate or methacrylic acid, increases the interlayer fracture toughness of the composites, although it causes a decrease in the interlayer shear strength. Thus, to create a three-dimensional self-healing fiber system that also provides high fracture toughness, filaments from ethylene-methacrylic acid copolymer were sewn into an epoxy carbon fiber laminate [
64]. The method of healing with thermoplastics requires the application of heat [
65].
Note that despite the development of self-healing methods, it would not hurt to have indicators in polymers and composite materials that can detect mechanical damage and the need for healing before the damage becomes catastrophic. This diagnostic is especially important for the method of intrinsic self-healing, in order to determine the moment at which it is necessary to stimulate the process with pressure and temperature. Research [
66] has offered an efficient way to measure the occurrence and propagation of damage by evaluating electrical resistance using embedded networks of carbon nanotubes. The applied deformation leads to an increase in the resistance in these materials due to the piezoresistance of individual NPs and an increase in the tunneling distance between the particles. Damage in the form of matrix cracking breaks electrical contacts, leading to an even more pronounced effect on the electrical resistance value.