Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Transcranial magnetic stimulation (TMS) is a noninvasive technique mainly used for the assessment of corticospinal tract integrity and excitability of the primary motor cortices. Motor evoked potentials (MEPs) play a pivotal role in TMS studies.
- multiple sclerosis
- TMS
- line navigation
- e-field navigation
1. Introduction
Multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system (CNS) of an unknown cause, characterized by demyelinating white matter lesions and neuronal degeneration [1]. The prevalence of MS in the world ranges from 5 to 300 per 100,000 people, and affects women more often [2]. Relapsing–remitting form of the disease (RRMS) is the most common form. The primary progressive form of the disease (PPMS) is significantly less common and occurs in 10% of people with MS (pwMS), while the further progression of the disease indicates the transition from the relapsing–remitting form to the secondary progressive form (SPMS).
The diagnosis of MS is based on laboratory findings (e.g., cerebrospinal fluid-specific bands), oligoclonal bands, and radiologic findings (e.g., magnetic resonance imaging [MRI ≥ 1.5 T or 3T] T2 lesions of the brain and spinal cord, lesions that increase gadolinium), including the application of the 2017 McDonald criteria and the 2021 MAGNIMS-CMSC-NAIMS recommendations [3,4]. The clinical status of disability is expressed through the Expanded Disability Status Scale (EDSS) [5,6], which assesses the status of functional systems including the pyramidal–corticospinal pathway (muscle strength, limb movement), cerebellum (balance, coordination), brainstem (speech, swallowing, nystagmus), sensory pathway (sensation), visual pathway (sight), bladder and bowel function, cognitive functions (memory), and ambulation (walking measured in meters). The key functional components of the EDSS, correlating with sustained disability progression, appear to be mostly pyramidal, followed by cerebellar and sensory functional systems [7].
Various quantitative measures (i.e., the number and volume of contrast-enhancing, the volumes of T2-hyperintense and T1-hypointense lesions, and brain volume changes), derived from conventional and advanced MRI methods, have been proposed as prognostic biomarkers for MS. However, correlations between different MRI indicators and EDSS are not satisfactory, and no specific MRI measure is used as a comprehensive prognostic imaging biomarker for MS [8,9,10].
Evoked potentials (EP) represent neurophysiological measures of signal conduction in the CNS in vivo, and are used to measure the impact of MS pathology on CNS function pathways correlating with clinical status [11]. Multimodal Eps, such as somatosensory evoked potentials (SEPs), visual evoked potentials (VEPs), and motor evoked potentials (MEPs), recorded as baseline (at diagnosis), have been shown to correlate with EDSS [12]. Recent findings suggest the likely application of TMS as a subclinical MEP test that could represent a biomarker of the degree of MS disability [13,14]. Current data suggest a connection between the pathophysiological mechanisms of MS (demyelination and loss of axons) and TMS neurophysiological measures (e.g., lower amplitudes and longer latencies of MEP responses from upper and lower limb muscles, elevated resting motor threshold (RMT), and changes in specific neurophysiological measures of excitation and inhibition) [15]. Furthermore, changes in cortical excitatory and inhibitory processes in MS, assessed with TMS, appear to be evident in early disease progression, during relapse, and later during disease progression [11,15,16]. In addition, changes in neurophysiological TMS measures are associated with the clinical characteristics of MS [14,15]. It has to be noted that MEPs acquired in TMS studies in MS subjects, mainly represent the marker of the integrity of the corticospinal tract (lateral funicle of the cord known as the lateral corticospinal tract) [17] and primary motor cortices (M1). Motor mapping can also demonstrate the presence of the ipsilateral MEP corticospinal tract projections reported in congenital pathologies, including hemiplegic cerebral palsy [18,19,20,21,22] and congenital mirror movements [18,23,24,25]; this is evident in progressive immune-mediated Rasmussen encephalitis, leading to unihemispheric brain atrophy [26] during intraoperative neurosurgical monitoring in patients [27], and in acquired lesions, such as during a cerebral stroke [28,29] or following hemispherectomy [28]. MEPs can also be recorded in the ipsilateral muscles of the upper extremities in healthy subjects [30]. Ipsilateral MEPs are thought to reflect the functional activity of the uncrossed lateral corticospinal tract from the ipsilateral hemisphere [31], may reflect the activation of the cortical–subcortical–spinal pathways [32], or may be due to the activation of the crossed corticospinal tract from the hemisphere contralateral to the target limb; this is due to the proximity of the M1 cortices for lower extremity muscle representation. The functional role of ipsilateral M1 areas in MS has been associated with an adaptive response to chronic CNS injury [33,34,35]. Overall, the TMS investigation of ipsilateral MEPs in MS has not been widely considered, due to the neurophysiological mechanisms still being unknown.
Concerning the clinical use and interpretation of MEPs in diagnosing and monitoring pwMS, TMS guidelines were established by Fernández et al. in 2013 [36], referring to the TMS. This mainly included the magnetic stimulator connected to a standard EMG unit, and was less connected to linenavigated TMS implementations.
2. Assessment of MEPs in Multiple Sclerosis
2.1. Targeting M1 with TMS without Navigation, Line-Navigated TMS and e-Field-Navigated TMS
TMS is a noninvasive technique mainly used for the evaluation of corticospinal tract integrity and the excitability of M1 cortices in MS. The basic principle of TMS can be explained by electromagnetic induction, generating a suprathreshold current in the brain. TMS devices consist of a few circular turns of copper wire, connected to the terminals of a large electrical capacitance via a switch. A large current (monophasic or biphasic pulse configuration) of several thousand Amps flows briefly through the wire coil for less than one millisecond. The current pulse produces a rapidly changing and brief magnetic field, with a field strength similar to the static field in an MRI scanner (1–2 T). Magnetic fields generate current in the brain tissue, according to Faraday’s law of electromagnetic induction (Figure 1).
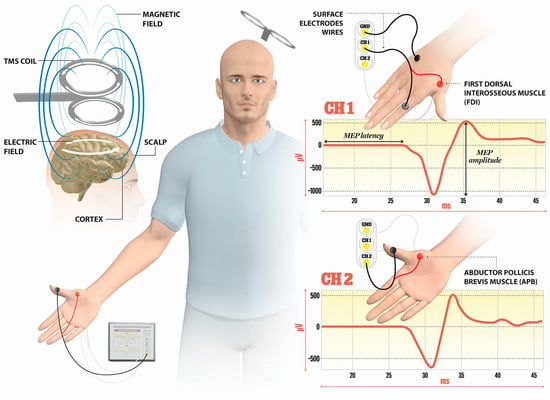
Figure 1. Illustration of the direction of current flows in a magnetic coil and the induced current in the brain tissue. An electric field is induced perpendicularly to the magnetic field. The magnetic coil is positioned over the M1 cortex, and surface electrodes are on the target muscles, here shown for the first dorsal interosseus (FDI) and abductor pollicis brevis (APB). Elicited MEP responses are detected at channels Ch1 for FDI, and Ch2 for APB muscle. The illustration is the property of the School of the Medicine University of Split, Department of Neuroscience, Laboratory for Human and Experimental Neurophysiology).
The TMS includes the magnetic stimulator connected to a standard EMG unit, with the positioning of the coil performed by using the external landmarks on the head. The determination of M1 representation for upper muscle is performed by the coil positioning; the coil is placed 5 cm lateral to the vertex along the auricular line and positioned by turning the coil approximately 45° to the parasagittal plane. In mapping the M1 representation for leg muscles, the coil is recommended to be placed over the vertex. Cervical stimulation is agreed upon by placing the coil above the C7 spinous process at the midline, or 2 cm lateral to the midline, while for the stimulation of lumbosacral roots, the coil is placed along the midline over the target vertebral body.
Line-navigated TMS is performed by placing a magnetic coil over the target area on the basis of the individual MRI image, with the maximal activation supposed to be located on the line that passes through the center of the coil perpendicular to the surface of the bottom of the coil; this is without the visualization of the spot of maximal stimulation if there is slight coil tilt [37]. Line-navigated TMS is susceptible to errors when the coil is not held continuously tangentially against the head.
E-field-navigated TMS computes the e-field maximum, where the cortex is best stimulated, online; it considers the geometry of the head, the magnetic coil shape, location, orientation, individual head shape, size, and the orientation of the cortical folds [37]. Navigated TMS combines TMS with 3D brain imaging, approximated with the spherical models, and comprises a magnetic stimulator, stereotactic camera, and integrated EMG system, including tracking tools (head tracker, coil tracker, digitizing pen). Prior to mapping M1 with navigated TMS, an MRI of the head for the subject is performed, including the MRI of the head and visible ears. After the co-registration of the subject, the reference anatomical spot for M1 for upper extremity representation is determined by the ‘‘omega knob’’ on axial MRI images, or a ‘‘hook structure’’ at the sagittal MRI [38]. The central sulcus is used as a landmark, while moving the coil in the anterior–posterior direction, to map the hot spot for M1 for the upper extremity muscle (i.e., abductor pollicis brevis, APB). When mapping the M1 for lower extremity muscles, the central sulcus is again followed as a landmark, with the posterior-to-anterior direction of the coil positioned medially over the vertex of the target hemisphere.
Line-navigated TMS and e-field-navigated TMS methods were compared in studies investigating MEPs; this was performed by stimulating the M1 area in tumor patients in preoperative settings [39], resulting in only a partial overlap in MEP maps while mapping M1 representation of upper and lower extremity muscles. The distances between the M1 motor hotspots between the two methods were 8.6 ± 4.5 mm on the contralesional hemisphere. Further, motor positive spots eliciting MEPs were significantly higher for e-field-navigated TMS, compared to line-navigated TMS. The lower rate of the positive motor hot spots detected with line-navigated TMS is probably due to a nonoptimal coil orientation and tilting with the decreased electric field at the cortex. In addition, the manual placing of the coil is more time-consuming in line-navigated TMS. Likewise, an e-field-navigated TMS can calculate and visualize the electric field online during the mapping procedure with its orientation and dose, allowing the continuous optimization of the coil positioning [39]. The final conclusions regarding the accuracy of the e-field-navigated TMS and line-navigated TMS methods are to be tested against the intraoperative golden standard direct electrical stimulation (DES) technique. Currently, e-field-navigated TMS systems have been evaluated in patients with tumors undergoing preoperative mapping of the M1 area and intraoperative DES procedures, showing a correlation between e-field-navigated TMS and DES [37].
Lastly, it is important to emphasize the variability in corticospinal excitability by mapping the M1 due to physical (tilt, location, intensity, and orientation of the coil) and physiological factors, in addition, interindividual anatomical differences in M1 that can be controlled by e-field-navigated TMS, including online calculation and visualization of an electric field [40,41], are mapped. The spatial accuracy of e-field-navigated TMS is approximately 2 mm [41], with location changes larger than 2 mm resulting in a variability of corticospinal excitability (i.e., changes in peak-to-peak MEP amplitude values), pointing to the fact that mapping of the integrity of the corticospinal tract is susceptible to small changes in physical parameters.
2.2. Neurophysiological Changes in the Central and Peripheral System in pwMS Investigated with TMS
The single-pulse TMS is applied for mapping the M1 and the integrity of the corticospinal tract by examining MEP outcome measures; this includes MEP latency (the transmission duration from the stimulating cortex to the onset of MEP in the EMG of the target muscle), MEP amplitude (peak-to-peak difference in MEP signal), the MEP input–output curve (I/O) (a sigmoid-shaped relation between the MEP amplitude at incremented TMS intensities), the central motor conduction time (CMCT) (the time it takes for the action potentials to travel from the site of cortical stimulation to the spinal neuron), the cortical silent period (CSP) (intracortical inhibition measure), or the resting motor threshold (RMT) (minimum intensity of stimulator output eliciting MEPs of 50 µV in at least ten trials in relaxing muscle) [17,42]. Further, short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and short-interval intracortical facilitation (SICF) can be explored if a paired-pulse TMS protocol is applied.
Recommendations for the clinical use of MEPs in MS are reported by Fernández et al. [36], and mainly discuss the application of TMS with no navigation for the use of MEP assessment in pwMS. The majority of reported studies, assessing the MEP in pwMS, used TMS apparatus with no navigation.
The findings for the neurophysiological assessment in MS, compared to healthy controls, include a prolongation in the MEP latency, an increase in the CMCT, and a decrease in the MEP amplitude, with still nonconclusive results related to RMT (findings point to be increased), CSP (findings point to be prolonged), and SICI (probably decreased) [17]. Two studies by Neva et al. [14] and Nantes et al. [43] used the TMS system with line navigation (neuronavigation software package by Rogue Research Inc., Canada), and a single group by Rogić Vidaković [44] used the e-field-navigated TMS to localize the M1 representation for upper and lower extremity muscles. So far, most of the MEP studies in MS have been conducted via TMS with no navigation, such as the study by Magstim, reporting the use of different coil types (circular, double-cone, figure-of-eight). Most of the studies included healthy controls (i.e., Pisa et al. [45]), or included the results of clinical samples of healthy controls in previously published studies. Recent reports tend to report the results of multimodal measures, including neurophysiological (MEP) assessment data, combined with MRI data on lesions, disease-related information, and clinical results of the neurological assessment (EDSS) [46].
This entry is adapted from the peer-reviewed paper 10.3390/s23010497
This entry is offline, you can click here to edit this entry!
