Ubiquitin is a highly-conserved small regulatory protein that has been found in almost all tissues of eukaryotic organisms. Ubiquitin was first identified in 1975. It performs its myriad functions through conjugation to a large range of target proteins. A variety of different modifications can occur. This discovery that ubiquitin can be attached to proteins and label them for destruction won the Nobel Prize for chemistry in 2004. The ubiquitin protein consists of 76 amino acids and has a molecular mass of about 8.5 kDa. Under the conditions where ATP provides energy, ubiquitin molecules bind to the target protein through the cascade catalytic reaction of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). The ubiquitinated target protein is recognized and degraded by 26S proteasome. Ubiquitination and deubiquitination are two popular ways for the post-translational modification of proteins. These two modifications affect intracellular localization, stability, and function of target proteins. The process of deubiquitination is involved in histone modification, cell cycle regulation, cell differentiation, apoptosis, endocytosis, autophagy, and DNA repair after damage. It is involved in the processes of carcinogenesis and cancer development. The deubiquitinating enzyme (DUB) function in head and neck squamous cell carcinoma (HNSCC) is discussed.
- HNSCC
- deubiquitinating enzyme
- cancer
1. The Role and Mechanism of Deubiquitinating Enzyme in Head and Neck Squamous Cell Carcinoma
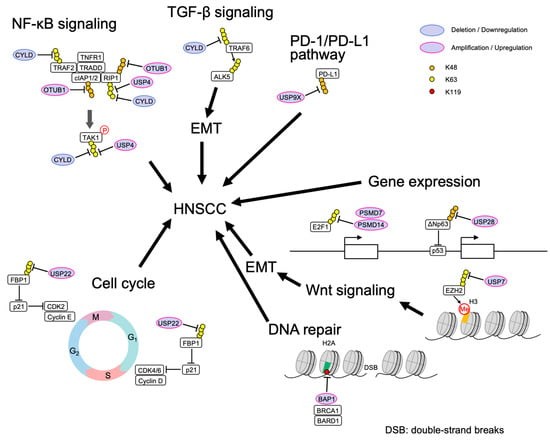
| DUB | Abnormal Regulation |
The Roles in HNSCC | Substrates | References |
|---|---|---|---|---|
| CYLD | mutation | removes K63-polyubiquitin and M1 linear-ubiquitin chains and inhibits NFκB signaling | RIP1, TRAF2, TRAF6, TAK1, NEMO | [4] |
| Low expression | promotes TGF-β signaling and cell invasion | ALK5 | [5] | |
| Low expression | removes K63-polyubiquitin, thereby inhibiting TGF-β signaling |
SMAD7 | [6][15] | |
| USP4 | overexpression | removes K63-polyubiquitin and promotes TNF-α induced apoptosis | RIP1 | [18] |
| USP7 | overexpression | promotes cell growth, cell migration, and invasion | EZH2 | [7][16] |
| USP9X | overexpression | deubiquitinates and stabilizes PD-L1 to promote cell proliferation |
PD-L1 | [19] |
| Low expression | mTOR pathway | [20] | ||
| USP22 | overexpression | associates with lymph node metastasis and histological grade | [21] | |
| USP28 | overexpression | inhibits p53 on the promoter of pro-apoptotic genes | Np63 | [8][9][10][11] |
| BAP1 | overexpression | deubiquitinates H2A at the DSB site suppresses transcription, and promotes DNA repair |
H2Aub(K119) | [22] |
| PSMD14 | overexpression | stabilizes E2F1, gives stemness to cells by SOX2 expression and Akt signal activation | E2F1 | [12] |
| PSMD7 | overexpression | a prognostic factor correlated with immune infiltration | [13] | |
| OTUB1 | overexpression | a risk factor | [4][14][17] |
2. The Role of Deubiquitinating Enzymes in Head and Neck Squamous Cell Carcinoma Cell Proliferation and Apoptosis
3. Relationship between Deubiquitinating Enzymes and Prognosis of Head and Neck Squamous Cell Carcinoma
4. The Role of Deubiquitinating Enzymes in Radiosensitivity of Head and Neck Squamous Cell Carcinoma
5. The Role of Deubiquitinating Enzymes in Immune Mechanisms of Head and Neck Squamous Cell Carcinoma
6. The Therapeutic Effect of Deubiquitinating Enzymes on Head and Neck Squamous Cell Carcinoma
This entry is adapted from the peer-reviewed paper 10.3390/ijms24010552
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709.
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on Head and Neck Cancer: Current Knowledge on Epidemiology, Risk Factors, Molecular Features and Novel Therapies. Oncology 2015, 89, 125–136.
- Morgan, E.L.; Chen, Z.; Van Waes, C. Regulation of NFkappaB Signalling by Ubiquitination: A Potential Therapeutic Target in Head and Neck Squamous Cell Carcinoma? Cancers 2020, 12, 2877.
- Shinriki, S.; Jono, H.; Maeshiro, M.; Nakamura, T.; Guo, J.; Li, J.D.; Ueda, M.; Yoshida, R.; Shinohara, M.; Nakayama, H.; et al. Loss of CYLD promotes cell invasion via ALK5 stabilization in oral squamous cell carcinoma. J. Pathol. 2018, 244, 367–379.
- Liu, S.; de Boeck, M.; van Dam, H.; Ten Dijke, P. Regulation of the TGF-beta pathway by deubiquitinases in cancer. Int. J. Biochem. Cell Biol. 2016, 76, 135–145.
- Zheng, N.; Chu, M.; Lin, M.; He, Y.; Wang, Z. USP7 stabilizes EZH2 and enhances cancer malignant progression. Am. J. Cancer Res. 2020, 10, 299–313.
- Sacco, J.J.; Coulson, J.M.; Clague, M.J.; Urbe, S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life 2010, 62, 140–157.
- Gatti, V.; Bernassola, F.; Talora, C.; Melino, G.; Peschiaroli, A. The Impact of the Ubiquitin System in the Pathogenesis of Squamous Cell Carcinomas. Cancers 2020, 12, 1595.
- Prieto-Garcia, C.; Hartmann, O.; Reissland, M.; Braun, F.; Fischer, T.; Walz, S.; Schulein-Volk, C.; Eilers, U.; Ade, C.P.; Calzado, M.A.; et al. Maintaining protein stability of ∆Np63 via USP28 is required by squamous cancer cells. EMBO Mol. Med. 2020, 12, e11101.
- Prieto-Garcia, C.; Tomaskovic, I.; Shah, V.J.; Dikic, I.; Diefenbacher, M. USP28: Oncogene or Tumor Suppressor? A Unifying Paradigm for Squamous Cell Carcinoma. Cells 2021, 10, 2652.
- Jing, C.; Duan, Y.; Zhou, M.; Yue, K.; Zhuo, S.; Li, X.; Liu, D.; Ye, B.; Lai, Q.; Li, L.; et al. Blockade of deubiquitinating enzyme PSMD14 overcomes chemoresistance in head and neck squamous cell carcinoma by antagonizing E2F1/Akt/SOX2-mediated stemness. Theranostics 2021, 11, 2655–2669.
- Zhang, S.; Yu, S.; Wang, J.; Cheng, Z. Identification of PSMD7 as a prognostic factor correlated with immune infiltration in head and neck squamous cell carcinoma. Biosci. Rep. 2021, 41, BSR20203829.
- Goncharov, T.; Niessen, K.; de Almagro, M.C.; Izrael-Tomasevic, A.; Fedorova, A.V.; Varfolomeev, E.; Arnott, D.; Deshayes, K.; Kirkpatrick, D.S.; Vucic, D. OTUB1 modulates c-IAP1 stability to regulate signalling pathways. EMBO J. 2013, 32, 1103–1114.
- Ge, W.L.; Xu, J.F.; Hu, J. Regulation of Oral Squamous Cell Carcinoma Proliferation Through Crosstalk Between SMAD7 and CYLD. Cell Physiol. Biochem. 2016, 38, 1209–1217.
- Zhang, M.J.; Chen, D.S.; Li, H.; Liu, W.W.; Han, G.Y.; Han, Y.F. Clinical significance of USP7 and EZH2 in predicting prognosis of laryngeal squamous cell carcinoma and their possible functional mechanism. Int. J. Clin. Exp. Pathol. 2019, 12, 2184–2194.
- Xu, L.; Li, Y.Y.; Zhang, Y.C.; Wu, Y.X.; Guo, D.D.; Long, D.; Liu, Z.H. A Novel Ferroptosis-Related Gene Signature to Predict Prognosis in Patients with Head and Neck Squamous Cell Carcinoma. Dis. Markers 2021, 2021, 5759927.
- Hou, X.; Wang, L.; Zhang, L.; Pan, X.; Zhao, W. Ubiquitin-specific protease 4 promotes TNF-alpha-induced apoptosis by deubiquitination of RIP1 in head and neck squamous cell carcinoma. FEBS Lett. 2013, 587, 311–316.
- Jingjing, W.; Wenzheng, G.; Donghua, W.; Guangyu, H.; Aiping, Z.; Wenjuan, W. Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med. 2018, 7, 4004–4011.
- Nanayakkara, D.M.; Nguyen, M.N.; Wood, S.A. Deubiquitylating enzyme, USP9X, regulates proliferation of cells of head and neck cancer lines. Cell Prolif. 2016, 49, 494–502.
- Dou, Y.; Lin, J.; Shu, H.; Jiang, N. Role of ubiquitin-specific peptidase 22 in carcinogenesis of human pharyngeal squamous cell carcinoma. Mol. Med. Rep. 2014, 10, 2973–2978.
- Liu, X.; Kumar, M.; Yang, L.; Molkentine, D.P.; Valdecanas, D.; Yu, S.; Meyn, R.E.; Heymach, J.V.; Skinner, H.D. BAP1 Is a Novel Target in HPV-Negative Head and Neck Cancer. Clin. Cancer Res. 2018, 24, 600–607.
- Glinsky, G.V.; Berezovska, O.; Glinskii, A.B. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J. Clin. Investig. 2005, 115, 1503–1521.
- Zhang, X.Y.; Varthi, M.; Sykes, S.M.; Phillips, C.; Warzecha, C.; Zhu, W.; Wyce, A.; Thorne, A.W.; Berger, S.L.; McMahon, S.B. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol. Cell 2008, 29, 102–111.
- Ling, S.; Li, J.; Shan, Q.; Dai, H.; Lu, D.; Wen, X.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol. Oncol. 2017, 11, 682–695.
- Zhuang, Y.J.; Liao, Z.W.; Yu, H.W.; Song, X.L.; Liu, Y.; Shi, X.Y.; Lin, X.D.; Zhou, T.C. ShRNA-mediated silencing of the ubiquitin-specific protease 22 gene restrained cell progression and affected the Akt pathway in nasopharyngeal carcinoma. Cancer Biol. Ther. 2015, 16, 88–96.
- Liu, H.; Liu, N.; Zhao, Y.; Zhu, X.; Wang, C.; Liu, Q.; Gao, C.; Zhao, X.; Li, J. Oncogenic USP22 supports gastric cancer growth and metastasis by activating c-Myc/NAMPT/SIRT1-dependent FOXO1 and YAP signaling. Aging (Albany NY) 2019, 11, 9643–9660.
- Atanassov, B.S.; Dent, S.Y. USP22 regulates cell proliferation by deubiquitinating the transcriptional regulator FBP1. EMBO Rep. 2011, 12, 924–930.
- Liu, W.; Wang, D.; Liu, L.; Wang, L.; Yan, M. miR-140 inhibits osteosarcoma progression by impairing USP22-mediated LSD1 stabilization and promoting p21 expression. Mol. Ther. Nucleic Acids 2021, 24, 436–448.
- Liu, T.; Liu, J.; Chen, Q.; Jin, S.; Mi, S.; Shao, W.; Kudo, Y.; Zeng, S.; Qi, G. Expression of USP22 and the chromosomal passenger complex is an indicator of malignant progression in oral squamous cell carcinoma. Oncol. Lett. 2019, 17, 2040–2046.
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178.
- Wang, M.L.; Panasyuk, G.; Gwalter, J.; Nemazanyy, I.; Fenton, T.; Filonenko, V.; Gout, I. Regulation of ribosomal protein S6 kinases by ubiquitination. Biochem. Biophys Res. Commun. 2008, 369, 382–387.
- Schwickart, M.; Huang, X.; Lill, J.R.; Liu, J.; Ferrando, R.; French, D.M.; Maecker, H.; O’Rourke, K.; Bazan, F.; Eastham-Anderson, J.; et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 2010, 463, 103–107.
- Panasyuk, G.; Nemazanyy, I.; Filonenko, V.; Gout, I. Ribosomal protein S6 kinase 1 interacts with and is ubiquitinated by ubiquitin ligase ROC1. Biochem. Biophys Res. Commun. 2008, 369, 339–343.
- Khan, O.M.; Carvalho, J.; Spencer-Dene, B.; Mitter, R.; Frith, D.; Snijders, A.P.; Wood, S.A.; Behrens, A. The deubiquitinase USP9X regulates FBW7 stability and suppresses colorectal cancer. J. Clin. Investig. 2018, 128, 1326–1337.
- Chen, X.; Wu, J.; Chen, Y.; Ye, D.; Lei, H.; Xu, H.; Yang, L.; Wu, Y.; Gu, W. Ubiquitin-specific protease 14 regulates cell proliferation and apoptosis in oral squamous cell carcinoma. Int. J. Biochem. Cell Biol. 2016, 79, 350–359.
- Nicholson, B.; Suresh Kumar, K.G. The multifaceted roles of USP7: New therapeutic opportunities. Cell Biochem. Biophys. 2011, 60, 61–68.
- Yamagishi, M.; Uchimaru, K. Targeting EZH2 in cancer therapy. Curr. Opin. Oncol. 2017, 29, 375–381.
- Tong, Z.T.; Cai, M.Y.; Wang, X.G.; Kong, L.L.; Mai, S.J.; Liu, Y.H.; Zhang, H.B.; Liao, Y.J.; Zheng, F.; Zhu, W.; et al. EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and Snail to inhibit E-cadherin. Oncogene 2012, 31, 583–594.
- Kim, J.; Kim, W.J.; Liu, Z.; Loda, M.; Freeman, M.R. The ubiquitin-specific protease USP2a enhances tumor progression by targeting cyclin A1 in bladder cancer. Cell Cycle 2012, 11, 1123–1130.
- da Silva, S.D.; Cunha, I.W.; Nishimoto, I.N.; Soares, F.A.; Carraro, D.M.; Kowalski, L.P.; Graner, E. Clinicopathological significance of ubiquitin-specific protease 2a (USP2a), fatty acid synthase (FASN), and ErbB2 expression in oral squamous cell carcinomas. Oral. Oncol. 2009, 45, e134–e139.
- Yu, H.; Pak, H.; Hammond-Martel, I.; Ghram, M.; Rodrigue, A.; Daou, S.; Barbour, H.; Corbeil, L.; Hebert, J.; Drobetsky, E.; et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. USA 2014, 111, 285–290.
- Molkentine, D.P.; Molkentine, J.M.; Bridges, K.A.; Valdecanas, D.R.; Dhawan, A.; Bahri, R.; Hefner, A.J.; Kumar, M.; Yang, L.; Abdelhakiem, M.; et al. p16 Represses DNA Damage Repair via a Novel Ubiquitin-Dependent Signaling Cascade. Cancer Res. 2022, 82, 916–928.
- Varilla, V.; Atienza, J.; Dasanu, C.A. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin. Biol. Ther. 2013, 13, 1241–1256.
- Klatka, J.; Hymos, A.; Szkatula-Lupina, A.; Grywalska, E.; Klatka, B.; Terpilowski, M.; Stepulak, A. T-Lymphocyte Activation Is Correlated With the Presence of Anti-EBV in Patients With Laryngeal Squamous Cell Carcinoma. In Vivo 2019, 33, 2007–2012.
- Whitehurst, C.B.; Ning, S.; Bentz, G.L.; Dufour, F.; Gershburg, E.; Shackelford, J.; Langelier, Y.; Pagano, J.S. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 2009, 83, 4345–4353.
- Dyson, O.F.; Pagano, J.S.; Whitehurst, C.B. The Translesion Polymerase Pol eta Is Required for Efficient Epstein-Barr Virus Infectivity and Is Regulated by the Viral Deubiquitinating Enzyme BPLF1. J. Virol. 2017, 91, e00600-17.
- Saito, S.; Murata, T.; Kanda, T.; Isomura, H.; Narita, Y.; Sugimoto, A.; Kawashima, D.; Tsurumi, T. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-kappaB signaling during productive replication. J. Virol. 2013, 87, 4060–4070.
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-kappaB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400.
- D’Arcy, P.; Wang, X.; Linder, S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol. Ther. 2015, 147, 32–54.
- Poondla, N.; Chandrasekaran, A.P.; Kim, K.S.; Ramakrishna, S. Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 2019, 52, 181–189.
- Singh, N.; Singh, A.B. Deubiquitinases and cancer: A snapshot. Crit. Rev. Oncol. Hematol. 2016, 103, 22–26.
- Mofers, A.; Pellegrini, P.; Linder, S.; D’Arcy, P. Proteasome-associated deubiquitinases and cancer. Cancer Metastasis Rev. 2017, 36, 635–653.
- Lee, H.R.; Choi, W.C.; Lee, S.; Hwang, J.; Hwang, E.; Guchhait, K.; Haas, J.; Toth, Z.; Jeon, Y.H.; Oh, T.K.; et al. Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat. Struct. Mol. Biol. 2011, 18, 1336–1344.
- Tian, Z.; D’Arcy, P.; Wang, X.; Ray, A.; Tai, Y.T.; Hu, Y.; Carrasco, R.D.; Richardson, P.; Linder, S.; Chauhan, D.; et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 2014, 123, 706–716.
- Woo, R.A.; Jack, M.T.; Xu, Y.; Burma, S.; Chen, D.J.; Lee, P.W. DNA damage-induced apoptosis requires the DNA-dependent protein kinase, and is mediated by the latent population of p53. EMBO J. 2002, 21, 3000–3008.
- An, J.; Mo, D.; Liu, H.; Veena, M.S.; Srivatsan, E.S.; Massoumi, R.; Rettig, M.B. Inactivation of the CYLD deubiquitinase by HPV E6 mediates hypoxia-induced NF-kappaB activation. Cancer Cell. 2008, 14, 394–407.
- Kiran, S.; Dar, A.; Singh, S.K.; Lee, K.Y.; Dutta, A. The Deubiquitinase USP46 Is Essential for Proliferation and Tumor Growth of HPV-Transformed Cancers. Mol. Cell 2018, 72, 823–835.e5.
- Shin, E.; Kim, J. The potential role of YAP in head and neck squamous cell carcinoma. Exp. Mol. Med. 2020, 52, 1264–1274.
- Zhang, W.; Luo, J.; Xiao, Z.; Zang, Y.; Li, X.; Zhou, Y.; Zhou, J.; Tian, Z.; Zhu, J.; Zhao, X. USP36 facilitates esophageal squamous carcinoma progression via stabilizing YAP. Cell Death Dis. 2022, 13, 1021.
