Eggs are a rich source of protein, minerals, lipids, and vitamins. Eggs are an essential source of bacterial microflora. Controlling antimicrobial resistance and reducing food loss and waste are essential for a sustainable future. To prevent spoilage and to preserve eggs, a variety of techniques, including thermal and non-thermal, are often used. The decontamination methods for egg preservation that have been applied are discussed. In previous studies, the initial contamination of the eggs varied from 2 to 9 log CFU per egg. Either thermal or non-thermal techniques resulted in reduced concentrations of Salmonella enteritidis, Salmonella typhimurium, and Escherichia coli, respectively, on the surface of the egg that ranged 0.62–5.9 log, 1.27–4.9 log, and 0.06–6.39 log, respectively, for the former, and being 1.2–7.8 log, 5.0–7.8 log, and 6.5–6.6 log, respectively, for the latter. Thermal approaches were more effective than the non-thermal approaches. Some of these methods had negative consequences on the egg’s functionality, while combination methods, such as thermoultrasonifcation (ozone-UV radiation or heat-ozone), mitigated these effects.
- egg
- food
- spoilage
1. Causes of Egg Spoilage and Methods of Preventing It
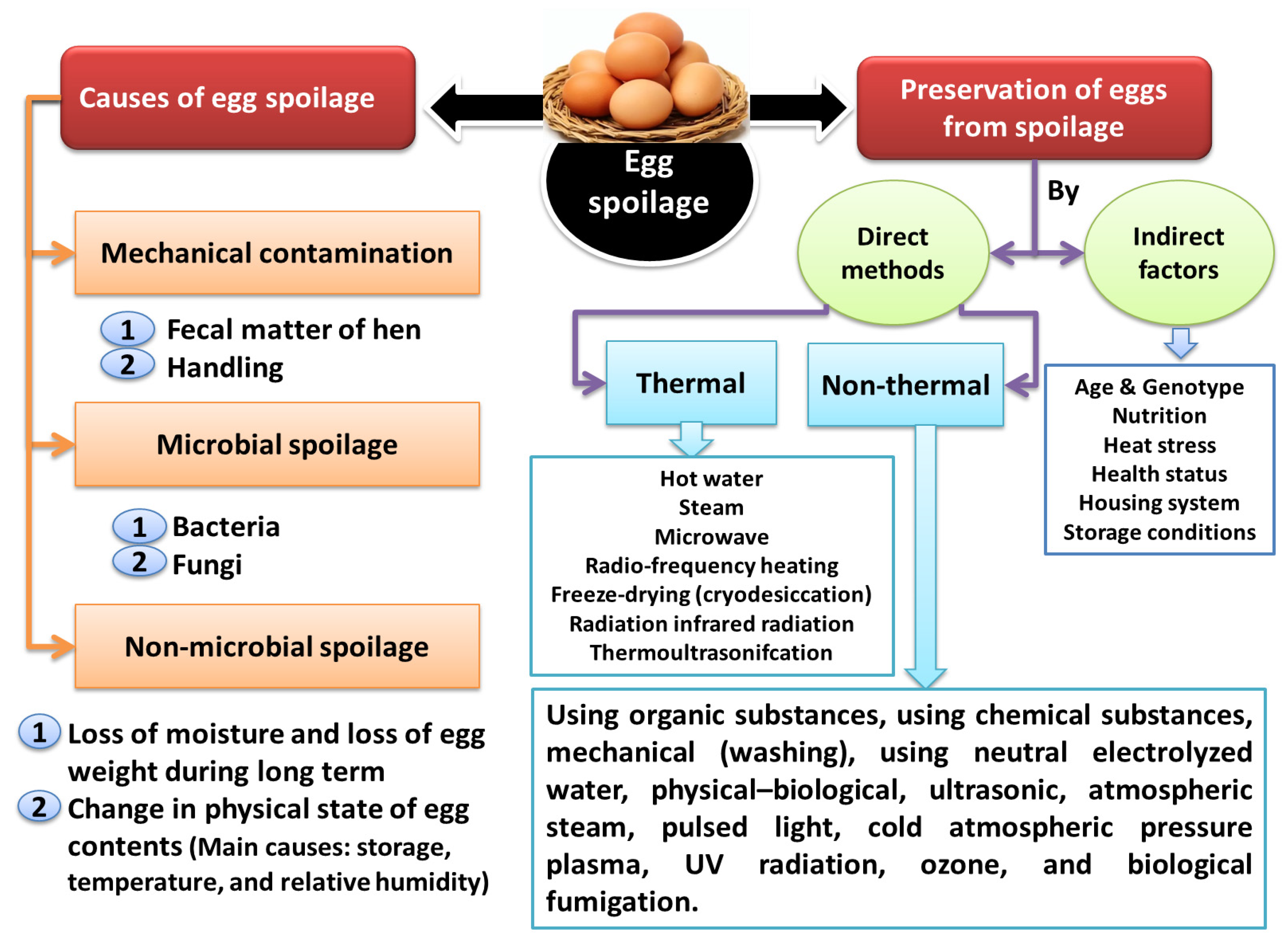
2. Direct Methods of Preventing Egg Spoilage
This entry is adapted from the peer-reviewed paper 10.3390/su15010464
References
- EFSA and ECDC. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406.
- Gast, R.K.; Regmi, P.; Guraya, R.; Jones, D.R.; Anderson, K.; Karcher, D.M. Contamination of eggs by Salmonella Enteritidis in experimentally infected laying hens of four commercial genetic lines in conventional cages and enriched colony housing. Poult. Sci. 2019, 98, 5023–5027.
- Eddin, A.S.; Ibrahim, S.A.; Tahergorabi, R. Egg quality and safety with an overview of edible coating application for egg preservation. Food Chem. 2019, 296, 29–39.
- McWhorter, A.R.; Chousalkar, K.K. Salmonella on Australian cage egg farms: Observations from hatching to end of lay. Food Microbiol. 2020, 87, 103384.
- Paramithiotis, S.; Drosinos, E.H.; Skandamis, P.N. Food recalls and warnings due to the presence of foodborne pathogens—A focus on fresh fruits, vegetables, dairy and eggs. Curr. Opin. Food Sci. 2017, 18, 71–75.
- Huang, X.; Hu, M.; Zhou, X.; Liu, Y.; Shi, C.; Shi, X. Role of yoaE Gene Regulated by CpxR in the Survival of Salmonella enterica Serovar Enteritidis in Antibacterial Egg White. mSphere 2020, 5, e00638-19.
- Raspoet, R.; Eeckhaut, V.; Vermeulen, K.; De Smet, L.; Wen, Y.; Nishino, K.; Haesebrouck, F.; Ducatelle, R.; Devreese, B.; Van Immerseel, F. The Salmonella Enteritidis TolC outer membrane channel is essential for egg white survival. Poult. Sci. 2019, 98, 2281–2289.
- Al-Bahry, S.N.; Mahmoud, I.Y.; Al-Musharafi, S.K.; Al-Ali, M.A. Penetration of spoilage and food poisoning bacteria into fresh chicken egg: A public health concern. Glob. J. Bio-Sci. Biotechnol. 2012, 1, 33–39.
- James, C.; Lechevalier, V.; Ketteringham, L. Surface pasteurisation of shell eggs. J. Food Eng. 2002, 53, 193–197.
- Techer, C.; Baron, F.; Jan, S. Microbial spoilage of eggs and egg products. World’s Poult. Sci. J. 2013, 69, 439–445.
- Liu, Y.-C.; Chen, T.-H.; Wu, Y.-C.; Lee, Y.-C.; Tan, F.-J. Effects of egg washing and storage temperature on the quality of eggshell cuticle and eggs. Food Chem. 2016, 211, 687–693.
- Muñoz, A.; Dominguez-Gasca, N.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.B. Importance of eggshell cuticle composition and maturity for avoiding trans-shell Salmonella contamination in chicken eggs. Food Control 2015, 55, 31–38.
- Knape, K.D.; Carey, J.B.; Ricke, S.C. Response of foodbornesalmonellaspp. marker strains inoculated on egg shell surfaces to disinfectants in a commercial egg washer. J. Environ. Sci. Health Part B 2001, 36, 219–227.
- Samiullah, S.; Chousalkar, K.; Roberts, J.; Sexton, M.; May, D.; Kiermeier, A. Effects of egg shell quality and washing on Salmonella Infantis penetration. Int. J. Food Microbiol. 2013, 165, 77–83.
- Al-Ajeeli, M.N.; Taylor, T.M.; Alvarado, C.Z.; Coufal, C.D. Comparison of eggshell surface sanitization technologies and impacts on consumer acceptability. Poult. Sci. 2016, 95, 1191–1197.
- Wang, H.; Slavik, M.E. Bacterial penetration into eggs washed with various chemicals and stored at different temperatures and times. J. Food Protec. 1998, 61, 276–279.
- Northcutt, J.K.; Musgrove, M.T.; Jones, D.R. Chemical Analyses of Commercial Shell Egg Wash Water. J. Appl. Poult. Res. 2005, 14, 289–295.
- Soljour, G.; Assanta, M.A.; Messier, S.; Boulianne, M. Efficacy of Egg Cleaning Compounds on Eggshells Contaminated with Salmonella enterica Serovar Enteritidis. J. Food Prot. 2004, 67, 706–712.
- Cheng, K.-C.; Dev, S.; Bialka, K.; Demirci, A. Electrolyzed oxidizing water for microbial decontamination of food. In Microbial Decontamination in the Food Industry; Demirci, A., Ngadi, M.O., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 563–591.
- Medina-Gudiño, J.; Rivera-Garcia, A.; Santos-Ferro, L.; Ramirez-Orejel, J.C.; Agredano-Moreno, L.T.; Jimenez-Garcia, L.F.; Paez-Esquiliano, D.; Martinez-Vidal, S.; Andrade-Esquivel, E.; Cano-Buendia, J.A. Analysis of Neutral Electrolyzed Water anti-bacterial activity on contaminated eggshells with Salmonella enterica or Escherichia coli. Int. J. Food Microbiol. 2020, 320, 108538.
- Huang, Y.-R.; Hung, Y.-C.; Hsu, S.-Y.; Huang, Y.-W.; Hwang, D.-F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345.
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332.
- Len, S.-V.; Hung, Y.-C.; Chung, D.; Anderson, J.L.; Erickson, M.C.; Morita, K. Effects of Storage Conditions and pH on Chlorine Loss in Electrolyzed Oxidizing (EO) Water. J. Agric. Food Chem. 2001, 50, 209–212.
- Ezazi, A.; Javadi, A.; Jafarizadeh-Malmiri, H.; Mirzaei, H. Development of a chitosan-propolis extract edible coating formulation based on physico-chemical attributes of hens’ eggs: Optimization and characteristics edible coating of egg using chitosan and propolis. Food Biosci. 2021, 40, 100894.
- Wardy, W.; Torrico, D.D.; Jirangrat, W.; No, H.K.; Saalia, F.K.; Prinyawiwatkul, W. Chitosan-soybean oil emulsion coating affects physico-functional and sensory quality of eggs during storage. LWT-Food Sci. Technol. 2011, 44, 2349–2355.
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154.
- Chen, Y.-W.; Ye, S.-R.; Ting, C.; Yu, Y.-H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768.
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2018, 83, 53–62.
- Oliveira, G.D.S.; dos Santos, V.M.; McManus, C. Propolis: Effects on the sanitisation of hatching eggs. World’s Poult. Sci. J. 2021, 78, 261–272.
- Drabik, K.; Batkowska, J.; Próchniak, T.; Horecka, B. Citric acid as a factor limiting changes in the quality of table eggs during their storage. Poult. Sci. 2021, 100, 100995.
- Soares, C.E.S.; Cartabiano-Leite, C.E.; Ferreira, W.X.; Maiorka, A.; Dahlke, F.; Scussel, V.M.; Lindner, J.D.D. Peracetic Acid: Effect on the Chicken Eggshell Cuticle and Decontaminating Action on Filamentous Fungi. Jokull J. 2021, 71, 82–96.
- Agregán, R.; Munekata, P.E.S.; Putnik, P.; Pateiro, M.; Kovačević, D.B.; Zavadlav, S.; Lorenzo, J.M. The Use of Novel Technologies in Egg Processing. Food Rev. Int. 2021; 1–21, in press.
- Ragni, L.; Berardinelli, A.; Vannini, L.; Montanari, C.; Sirri, F.; Guerzoni, M.E.; Guarnieri, A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J. Food Eng. 2010, 100, 125–132.
- Dasan, B.G.; Yildirim, T.; Boyaci, I.H. Surface decontamination of eggshells by using non-thermal atmospheric plasma. Int. J. Food Microbiol. 2018, 266, 267–273.
- Moritz, M.; Wiacek, C.; Weihe, T.; Ehlbeck, J.; Weltmann, K.; Braun, P.G. Effect of cold atmospheric pressure plasma treatment of eggshells on the total bacterial count inoculated Salmonella Enteritidis and selected quality parameters. Plasma Process. Polym. 2020, 18, 2000061.
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2014, 10, 1–11.
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.; Gilmore, B. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324.
- Perry, J.J.; Yousef, A.E. Decontamination of Raw Foods Using Ozone-Based Sanitization Techniques. Annu. Rev. Food Sci. Technol. 2011, 2, 281–298.
- Soares, C.E.D.S.; Leite, C.E.C.; Dahlke, F.; Maiorka, A.; Miotto, M.; Scussel, V.; Lindner, J.D.D. Antifungal Action of Ozone on Chicken Eggshell Cuticles: A Preliminary Study. Ozone Sci. Eng. 2021, 44, 407–412.
- Braun, P.; Fernández, N.; Fuhrmann, H. Investigations on the Effect of Ozone as a Disinfectant of Egg Surfaces. Ozone Sci. Eng. 2011, 33, 374–378.
- Yüceer, M.; Aday, M.S.; Caner, C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agric. 2015, 96, 2755–2763.
- Lasagabaster, A.; Arboleya, J.C.; de Marañón, I.M. Pulsed light technology for surface decontamination of eggs: Impact on Salmonella inactivation and egg quality. Innov. Food Sci. Emerg. Technol. 2011, 12, 124–128.
- Wang, B.; Wei, W.; Aputexiakere, J.; Li, Y.; Ma, H. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2021, 137, 108411.
- John, D.; Ramaswamy, H.S. Pulsed light technology to enhance food safety and quality: A mini-review. Curr. Opin. Food Sci. 2018, 23, 70–79.
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13.
- Sert, D.; Aygun, A.; Demir, M. Effects of ultrasonic treatment and storage temperature on egg quality. Poult. Sci. 2011, 90, 869–875.
- Rodriguez-Romo, L.A.; Yousef, A.E. Inactivation of Salmonella enterica serovar Enteritidis on shell eggs by ozone and UV radiation. J. Food Prot. 2005, 68, 711–717.
- Aygun, A.; Sert, D. Effects of vacuum packing on eggshell microbial activity and egg quality in table eggs under different storage temperatures. J. Sci. Food Agric. 2013, 93, 1626–1632.
- Suwannarach, N.; Kaewyana, C.; Yodmeeklin, A.; Kumla, J.; Matsui, K.; Lumyong, S. Evaluation of Muscodor cinnamomi as an egg biofumigant for the reduction of microorganisms on eggshell surfaces and its effect on egg quality. Int. J. Food Microbiol. 2017, 244, 52–61.
- Mukhopadhyay, S.; Ukuku, D.O. The role of emerging technologies to ensure the microbial safety of fresh produce, milk and eggs. Curr. Opin. Food Sci. 2018, 19, 145–154.
- Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. Reducing Risk of Salmonellosis through Egg Decontamination Processes. Int. J. Environ. Res. Public Health 2017, 14, 335.
- Lechevalier, V.; Guérin-Dubiard, C.; Anton, M.; Beaumal, V.; Briand, E.D.; Gillard, A.; Le Gouar, Y.; Musikaphun, N.; Tanguy, G.; Pasco, M.; et al. Pasteurisation of liquid whole egg: Optimal heat treatments in relation to its functional, nutritional and allergenic properties. J. Food Eng. 2016, 195, 137–149.
- Uysal, R.S.; Boyacı, I.H.; Soykut, E.A.; Ertaş, N. Effects of heat treatment parameters on liquid whole egg proteins. Food Chem. 2017, 216, 201–208.
- Yang, Y.; Geveke, D.J. Shell egg pasteurization using radio frequency in combination with hot air or hot water. Food Microbiol. 2019, 85, 103281.
- Zion, B.; Gollop, R.; Barak, M.; Saldinger, S.S.; Arbel, A. External disinfection of shell eggs using steam in a Thermal Trap. Food Control 2021, 127, 108135.
- Lakins, D.; Alvarado, C.; Thompson, L.; Brashears, M.; Brooks, J.C.; Brashears, M.M. Reduction of Salmonella Enteritidis in Shell Eggs Using Directional Microwave Technology. Poult. Sci. 2008, 87, 985–991.
- Cabeza, M.C.; Garcia, M.L.; Hoz, L.D.; Cambero, I.; Ordontez, J.A. Thermoultrasonication Eliminates Salmonellae from Intact Eggshells without Changing the Functional Properties of their Components. J. Food Sci. 2006, 70, m292–m295.
- de Souza Aquino, J.; da Silva, J.A.; Prado, J.P.; de Oliveira Cavalheiro, J.M. Análise dos constituintes de gema de ovo de avestruz desidratada por meio de duas metodologias de secagem. Rev. Inst. Adolfo Lutz. 2008, 67, 190–195.
