The tumor is an uncontrolled growth of tissue that can be localized (benign) or possesses the capability of metastasis (malignant). The conventional methods of tumor diagnosis, such as acupuncture, endoscopy, and histopathology, and treatment methods, such as injections, chemotherapy, surgery, and radiotherapy, are invasive, expensive, and pose severe safety and management issues for the patients. Microneedle technology is minimally invasive, self-administrable, bypasses the first-pass effect, effectively delivers chemotherapeutics and drugs at low doses, and provides drug diffusion into the tumor areas, thus, overcoming the drawbacks of conventional delivery systems.
- microneedles
- tumor therapy
- tumor diagnosis
1. Introduction
Microneedles in Cancer Therapy and Diagnosis
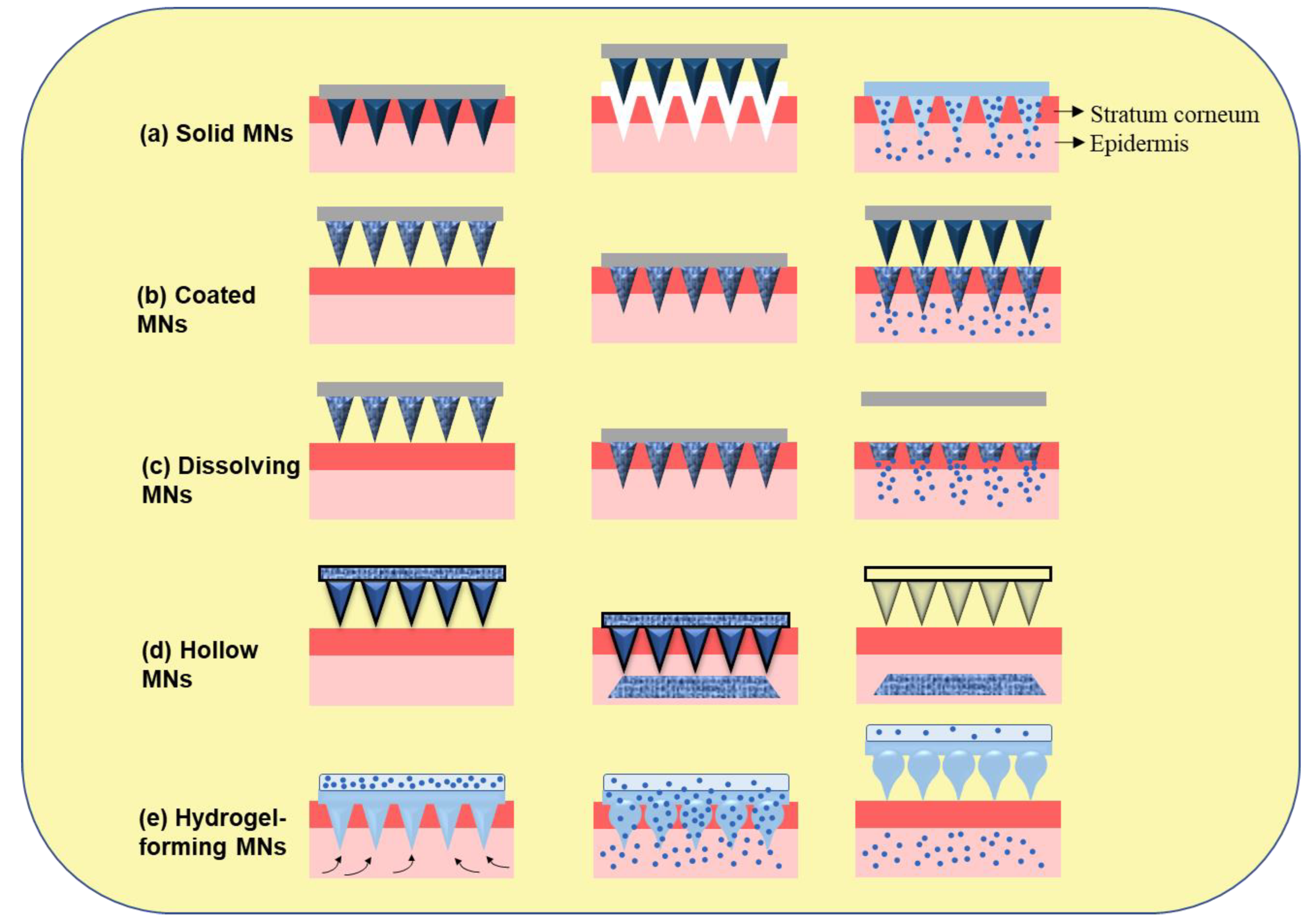
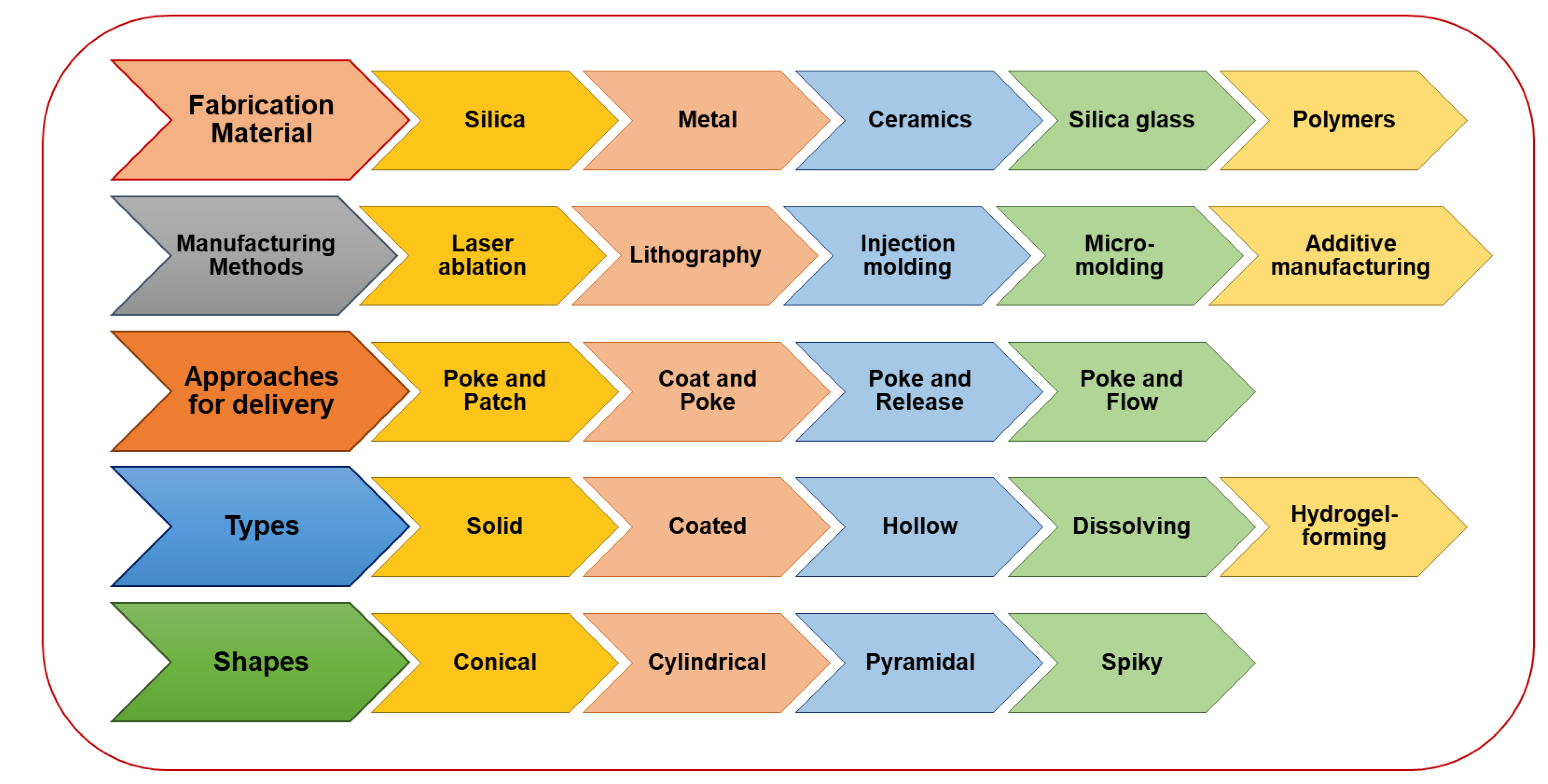
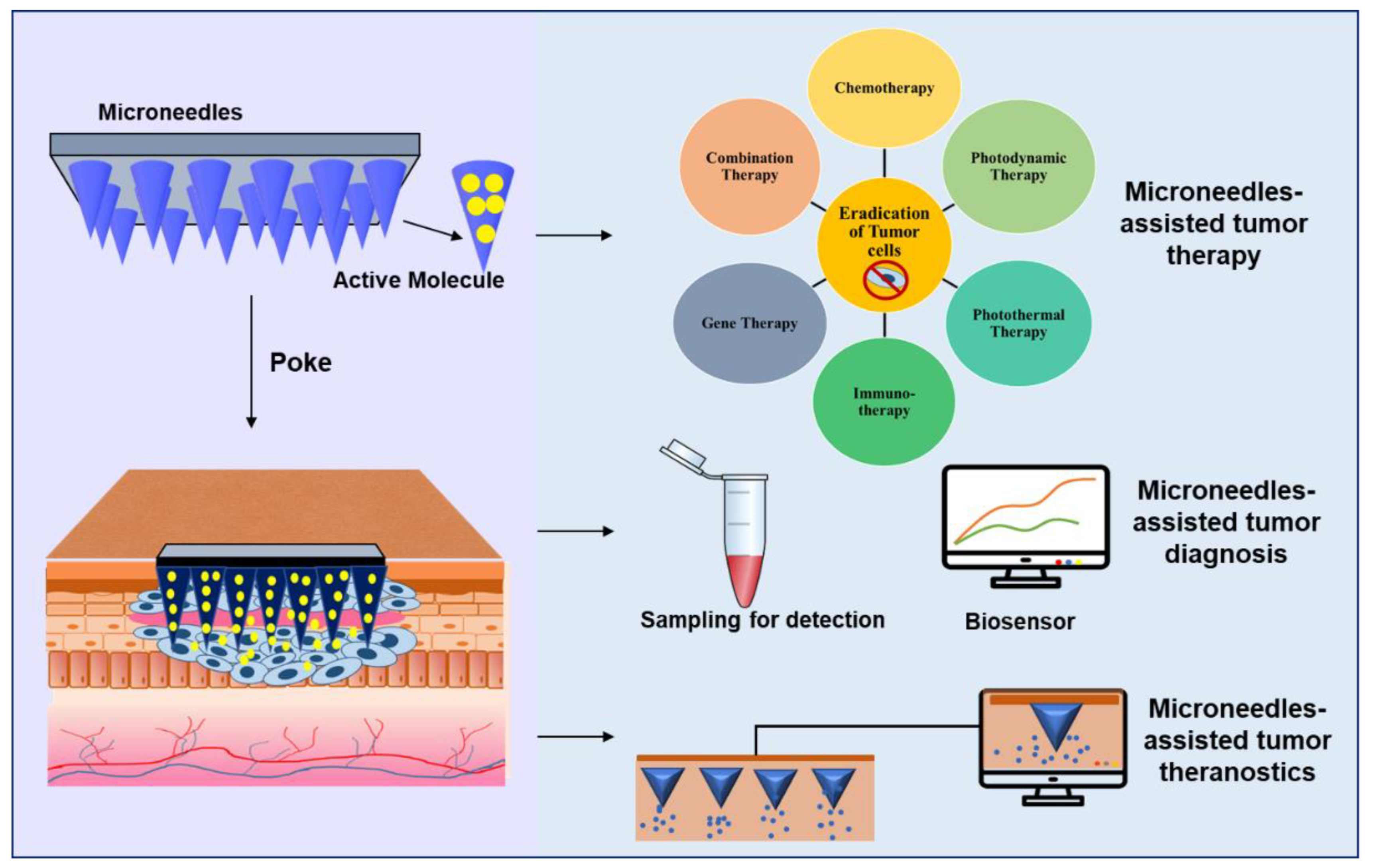
Figure 3. Microneedles-assisted tumor therapy, diagnosis, and theranostics.
3. Conclusion
MNs-assisted monotherapy and combination therapies for tumors overcome the majority of drawbacks, such as the effective and localized delivery of chemotherapeutic agents at low doses; site-specific delivery, preventing damage to healthy tissues; and the rapid and effective delivery of nanoparticles, liposomes, and other delivery agents, which are difficult to administer by conventional routes. MNs are also effectively used as diagnostic tools to bio-sample blood or Interstitial Fluid and biosensing various cancer biomarkers, enabling rapid and early detection which can allow the timely initiation of treatment. MNs are also applied as theranostic tools that combine therapy and diagnosis, such as tumor imaging-guided therapies, and are ideal for personalized tumor treatment. Nanocarrier systems with pH-responsive and enzyme-responsive properties were also successfully incorporated with MNs. To cater to the requirements of controlled release, MNs were designed as multi-layered or core-shell structures, which shows their practical and wide applicability with innovative designs [9]. Therefore, MNs are an emerging diagnostic and drug delivery system with unique features that contribute significantly to cancer and tumor therapy and diagnosis, which is challenging by conventional methods.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15010014
References
- Beale, J.M.; Block, J.H.R. Wilson and Gisvold’s Textbook of Organic Medicinal And Pharmaceutical Chemistry, 12th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 355–412.
- Miskawaan Integrative Cancer Care Global Cancer Facts and Figures 2021. Available online: https://www.miskawaanhealth.com/cancer/global-cancer-statistics/ (accessed on 24 June 2022).
- WHO Cancer. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 24 June 2022).
- Hejmady, S.; Pradhan, R.; Alexander, A.; Agrawal, M.; Singhvi, G.; Gorain, B.; Tiwari, S.; Kesharwani, P.; Dubey, S.K. Recent advances in targeted nanomedicine as promising antitumor therapeutics. Drug Discov. Today 2020, 25, 2227–2244.
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Siddaramaiah; Gowda, D.V.; Moin, A. Microneedles drug delivery systems for treatment of cancer: A recent update. Pharmaceutics 2020, 12, 1101.
- Alimardani, V.; Abolmaali, S.S.; Tamaddon, A.M.; Ashfaq, M. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. Transl. Res. 2021, 11, 788–816.
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Microneedle-based delivery devices for cancer therapy: A review. Pharmacol. Res. 2019, 148, 104438.
- Foye, W.O. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 1199–1266.
- Lin, S.; Cao, Y.; Chen, J.; Tian, Z.; Zhu, Y. Recent advances in microneedles for tumor therapy and diagnosis. Appl. Mater. Today 2021, 23, 101036.
- Rapalli, V.K.; Mahmood, A.; Waghule, T.; Gorantla, S.; Dubey, S.K.; Alexander, A.; Singhvi, G. Revisiting techniques to evaluate drug permeation through skin. Expert Opin. Drug Deliv. 2021, 18, 1829–1842.
- Jain, R.; Sarode, I.; Singhvi, G.; Dubey, S.K. Nanocarrier Based Topical Drug Delivery- A Promising Strategy for Treatment of Skin Cancer. Curr. Pharm. Des. 2020, 26, 4615–4623.
- Aldawood, F.K.; Andar, A.; Desai, S. A comprehensive review of microneedles: Types, materials, processes, characterizations and applications. Polymers 2021, 13, 2815.
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258.
- Priya, S.; Singhvi, G. Microneedles-based drug delivery strategies: A breakthrough approach for the management of pain. Biomed. Pharmacother. 2022, 155, 113717.
- Dabholkar, N.; Gorantla, S.; Waghule, T.; Rapalli, V.K.; Kothuru, A.; Goel, S.; Singhvi, G. Biodegradable microneedles fabricated with carbohydrates and proteins: Revolutionary approach for transdermal drug delivery. Int. J. Biol. Macromol. 2021, 170, 602–621.
