The wide application of fertilizers in tile-drained agricultural systems commonly results in the loss of phosphorus (P) to the underground tile drains during precipitation events and emission to downstream surface waters. As different catchments are subject to local geological and climatic conditions, among other factors, it is observed a large spatiotemporal variation in the dynamics of P emissions, including the load and dominant form (i.e., particulate or dissolved). In-farm practices may be able to mitigate this problem to some extent (e.g. by optimizing the P application), but may not suffice in areas saturated with P. Therefore, systems designed to reduce the excess P loads at relatively low costs and located at the edge of tile-drained catchments have been tested and implemented. This has been done by increasing the hydraulic residence time of the drainage discharge, allowing sedimentation of particles and biogeochemical processes between the water, soil/sediments and biota to occur; by trapping P at the bank of watercourses; and by enhancing the retention of dissolved P with filters rich in P sorbents. The retention of P by these measures, however, can be rather variable and largely depends on the catchment conditions. Thus, a series of considerations, e.g., in regards to design parameters, long-term stability of the P retained and retention of different P forms, must be taken into account, including major constraints (e.g., use of agricultural land), to ensure successful application of edge-of-field measures and achieve the desired cost-efficiency.
- edge-of-field technologies
- agricultural drainage water
- phosphorus retention
- constructed wetland
- restored wetland
- vegetated buffer strip
- integrated buffer zone
- filter material
Phosphorus emission from agriculture
The widespread use of fertilizers in agricultural fields has commonly led to the accumulation of phosphorus (P) in the soil followed by loss during precipitation events[1]. The P loads eventually reach downstream surface waters and may cause eutrophication[2]. This is particularly the case in tile-drained agricultural areas, where the excess P in the soil is rapidly transported in drainage networks (Figure 1).
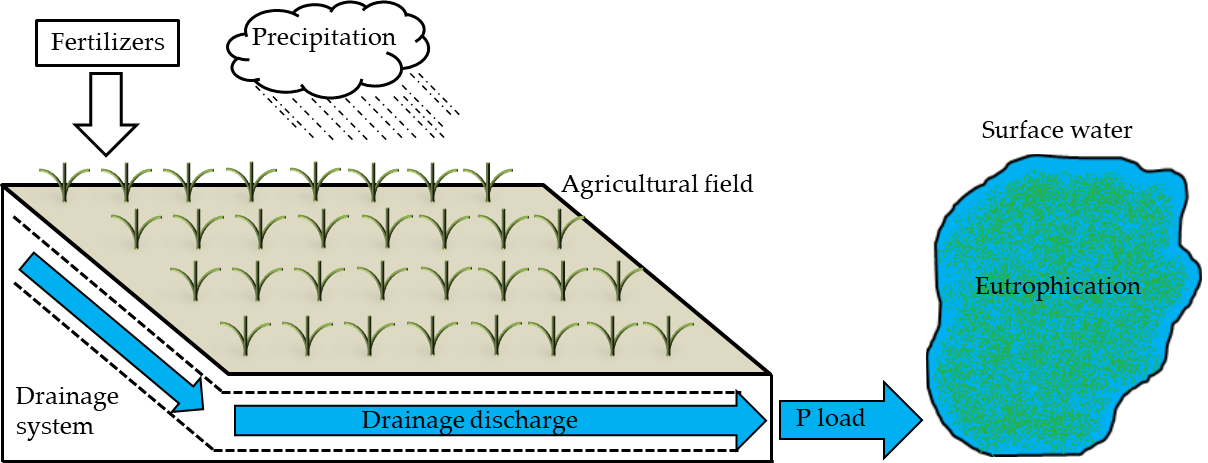
Figure 1. Schematic of phosphorus (P) transport from a tile-drained agricultural area to a downstream surface water, causing eutrophication. Adapted from Mendes[3].
The P load and dominant P form in tile drains vary in different agricultural catchments according to local conditions (e.g., geology, climate and agricultural practices)[1]. Moreover, large variations can also occur in the same catchment mainly due to seasonal variations in precipitation, resulting in base and peak-flow events[4]. The leaching of P to tile drains occurs in dissolved and particulate forms, and the dominant form can vary depending on the soil pH and redox conditions as well as the P sorption capacity, which all influence sorption reactions of P to its sorbents (e.g., iron, aluminum and calcium)[5]. In addition, finer-textured soils may favor the loss of particles and associated P[6], as well as sorption to dissolved P owing to larger surface area[7].
In-farm attempts to minimize P loss
Agricultural practices have a great effect on the loss of P. This reflects mainly on the application and management of fertilizers[8]. Therefore, attempts to optimize crop uptake with the amount of P inputs in the field are essential to minimize P loss[9]. Other practices may include tillage[10], catch crops[11], soil liming[9] and controlled drainage[12]. Furthermore, strategies to reduce the P loss at agricultural catchments may target the leaching of P into tile drains by increasing the P sorption capacity of the subsoils (e.g., by mixing with materials rich in P sorbents)[13]. However, the effect of in-farm practices may be limited by long-term loss of P in soils with considerable P concentrations. Moreover, freezing-thaw cycles and heavy precipitation events promote leaching of P in an annual basis[14][15]. Therefore, additional measures may be needed to ensure that the P load transported through drainage networks is reduced prior to discharging into surface waters.
Mitigation measures at the edge of the catchment
Agricultural areas where in-farm practices are not enough to reduce the P loads to acceptable levels normally need measures implemented at the edge of the catchment. This is commonly the case when P loss to the tile drains is considerably high (i.e., critical source areas). Currently recognized and used measures or technologies include constructed wetlands, restored wetlands, vegetated buffer strips and filter materials. These systems are implemented so as to receive the drainage discharge at the outlet of the main drainage pipe and reduce the P load to surface waters through mechanisms deemed low cost, e.g., sedimentation, sorption and biological uptake (Figure 2).
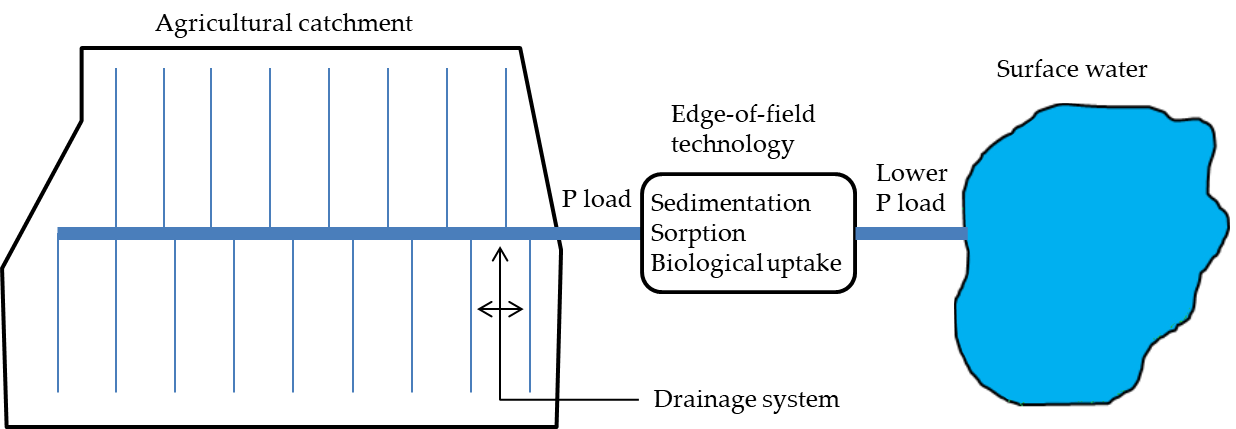
Figure 2. Schematic of an edge-of-field technology receiving drainage discharge from an agricultural catchment to reduce the excess phosphorus (P) load by intrinsic retention mechanisms prior to reaching a downstream surface water.
The effect of edge-of-field technologies on reducing the P loads, however, can be rather difficult to predict, and is in many cases variable, especially when the drainage discharge is event-driven or typically has seasonal oscillations. Moreover, it is observed that the catchment characteristics (e.g., P load and dominant P form) play a large role on the performance of these systems. Therefore, it is important that these are designed so as to achieve acceptable P retention according to local conditions, which would consequently improve the cost-efficiency.
Retention of P by increasing the hydraulic residence time
One way to reduce the P load at the edge of the catchment is by allowing the drainage discharge to flood over a certain area in order to increase the hydraulic residence time (HRT) and favor natural retention mechanisms. This is the case for constructed and restored wetlands, which replicate the P retention processes of natural wetlands (Figure 3a). Herein, sedimentation of particulate P is especially promoted, as the water flow decreases at the inlet of these systems, allowing particles to settle on the upper sediments[16]. Therefore, it is expected that a layer of deposited material containing most of the P retained will form and develop over time, resulting in long-term P retention[17]. Sorption and biological uptake of dissolved P also occur, although generally to a lesser extent, as transport of dissolved P to reactive sites in the soil or plant roots depends largely on diffusion regulated by concentration gradients[18]. Therefore, satisfactory retention of dissolved P normally requires long HRTs–which can cause great variability in retention under different HRTs–in addition to sufficient amounts of P sorbents. Moreover, retention of dissolved P can be limited by the mineralization of previously assimilated P by biota during the senescence period[19], or by the release of P bound to redox-sensitive sorbents (e.g., iron) under reducing conditions[16]. Therefore, it is important that these systems ensure not only adequate HRT for P retention, but also biogeochemical stability and the presence of oxidizing conditions for the P retained.
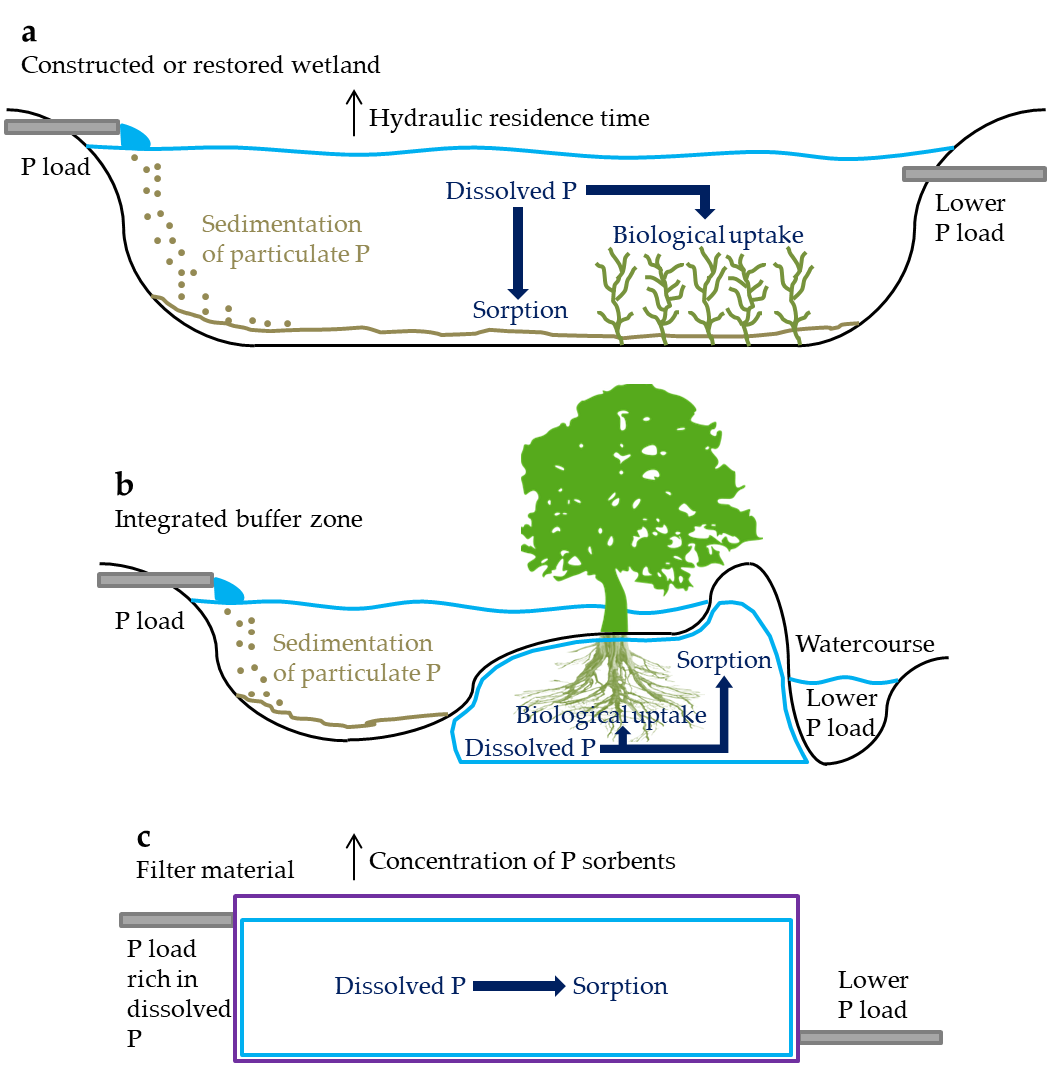
Figure 3. Schematic of reduction processes for phosphorus (P) load from agricultural drainage discharge in a constructed or restored wetland (a); integrated buffer zone (b), adapted from Zak et al.[20]; and filter material (c). The water, sediment, soil and filter compartments are indicated by the blue, brown, black and purple lines, respectively. The water flow direction is from left to right.
Constructed wetlands and their applications have been particularly studied, and it is found that these are generally effective as P sinks[21][22][23], although net P export has also been observed in some cases[24][25]. Large variations in HRT normally affect the performance, and systems with more stable hydrological regime tend to demonstrate better and consistent results[26]. However, systems receiving increasing P loads tend to present higher mass P retention[27], even though this may reflect on lower HRTs. In this case, the P retention mechanisms are promoted by the increasing P inputs. Decreasing the HRT to a critical threshold, on the other hand, may prevent a significant fraction of the P load to be retained, and result in lower percentage P retention[26]. Variations in HRT may thus have a marked effect on the retention efficiency (%). Therefore, it is important that the water flow is not high enough to substantially decrease the contact time of the drainage discharge with these systems (i.e., the HRT) and lessen the retention of P.
Wetland restoration occurs in areas where a natural wetland existed and was drained for the expansion of agriculture[16]. These areas were thus subject to crop rotations and the application of fertilizers. Therefore, it is common that restored wetlands initiate operation with high P contents in their topsoil layers, which normally result in low availability of P sorption sites. Increasing the HRT for the drainage discharge in these systems, therefore, may support P desorption and release from the soil to the water column upon re-flooding owing to a P concentration gradient, emergence of reducing conditions[28] and mineralization of organic P from previous crop rotations[29]. These likely effects indicate that the performance of restored wetlands in the retention of P can be rather unpredictable, and that these systems are a potential source of dissolved P[29]. In line with this, P retention in these systems may be more sensitive to variations in HRT in comparison to constructed wetlands.
Protection along the bank of watercourses
A common measure to prevent the discharge of high P loads into watercourses flowing through agricultural fields is the use of vegetated buffer strips. These consist of a variety of plants, including trees, bushes and grass located between agricultural catchments and downstream watercourses such as streams and rivers[30]. Therein, the excess P load is expected to be retained by plant uptake in the root zone, entrapment of particulate P into the soil matrix or vegetation, and sorption of dissolved P to reactive sites in the soil profile[30][31]. There is a vast documentation of the effect of these systems on the treatment of P from agricultural surface runoff. In tile-drained catchments, however, the tile drains commonly lead the drainage discharge across the systems, skipping the P retention mechanisms and resulting in no or little P retention[30]. Thus, an alternative system design called integrated buffer zone, which combines a ditch-like pond with a vegetated filter bed, has been tested to receive and treat agricultural drainage discharge[20] (Figure 3b). The initial results are promising, with significant reductions on the P loads and effective retention of dissolved P.
Enhancing sorption of dissolved P
Retention of dissolved P can be limited or highly variable by the measures described above, as retention mechanisms for particulate P are generally more efficient. Therefore, alternative systems have been tested to target P loads rich in dissolved P. These include a series of porous filter materials with different retention capacities for P, in which the primary retention mechanism is sorption (Figure 3c). Therefore, the materials must be rich in P sorbents and have appropriate hydraulic conductivity so as to allow the drainage discharge to flow through the system and promote the retention of P[32]. Furthermore, these must ensure low P desorption rates.
Commonly used filters contain high contents of iron and aluminum, in which sorption of P onto the surface material occurs, or calcium, in which P retention occurs through precipitation[33]. Batch and column experiments have demonstrated that the former filters are generally more efficient due to fast ligand exchange with P, resulting in higher retention and lower desorption rate[34][35][36]. Thus, these have been recommended for application in the treatment of drainage discharge, either as single systems or paired with woodchip bioreactors, which target the reduction of nitrogen loads. Calcium-based filters, on the other hand, may be recommended when the HRT is long enough to allow precipitation with P at an acceptable rate[37] and when the drainage discharge has a basic pH[38], which promotes calcium solubility. Finally, intragranular P diffusion caused by P concentration gradients between mobile and immobile pore regions was found to be an important retention mechanism which enhances P sorption in filter materials[35].
Local catchment conditions also influence the level of P retention in filter materials. Systems receiving increasing hydraulic loads and P concentrations tend to show higher mass P retention, as this enhances the sorption rate of P[37]. On the other hand, this may decrease the percentage P retention, as the HRT would be shorter. As filter materials are relatively small systems, marked variations in HRT may be expected during operation, resulting in varying P retention.
Considerations in the implementation of edge-of-field technologies
In order to successfully reduce the P loads at the edge of tile-drained catchments, it is fundamental to evaluate whether the implemented technologies are cost-effective. This can be ensured, for example, by targeting local and specific catchment conditions with well-designed systems. These can take into account appropriate sizing and configuration so as to allow sufficient HRT and proper hydraulic efficiency, i.e., the water flow distribution across the system, which affects the level of P mixing. In the case of constructed and restored wetlands, the aspect ratio of length to width and location of the inlet and outlet are generally relevant when addressing design considerations, as these affect the hydraulic efficiency[39]. Retention of P in vegetated buffer strips, in turn, normally enhances the larger the width of the system, with greater effects on steeper slopes[40]. In regards to filter materials, the mass and composition of the material are relevant when estimating the P retention rate and efficiency (%) desired[37]. Iron and aluminum-based filters are recommended in tile-drained agricultural catchments in which the water flow is considerably variable and high owing to fast sorption reactions, whereas filters based on calcium may be implemented when the hydrological regime is considered stable and HRTs are sufficiently long to allow precipitation with P and acceptable retention[37]. Thus, the use of the latter filters can be restricted. Moreover, the pH of the discharge may also influence the choice of the filter material, where acid and basic discharges favor P retention in iron and aluminum, and calcium-based filters, respectively[38]. Finally, it is important to ascertain that the concentration of dissolved P in the drainage discharge is not sufficiently low to induce P desorption from the filter to be implemented as a result of P concentration gradients.
The load of P sorbents in the drainage discharge is also relevant when implementing edge-of-field technologies, especially in constructed and restored wetlands, which are prone to P saturation in the long-term[17]. In this context, a consistent load would ensure the availability of P sorption sites in the soil and sediments, and maintain the P stability, resulting in long-term P retention.
Despite the documented efficiency of edge-of-field technologies in the retention of P, these are expected to decrease the performance in the long-term due to accumulation of P and saturation of P sorption sites, which decrease the stability of P and enhance the chance of P release. In constructed and restored wetlands, this particularly occurs in the sediment layer, while filter materials may no longer be able to retain P after being spent. The system design and catchment conditions fundamentally determine the lifetime or operating time of the system. Therefore, maintenance operations can be expected within a timeframe, and normally include harvesting of the vegetation, removal of the sediment layer and replacement of the spent filter material. The idea is to remove or replace the saturated P storage compartment of the system to enhance P retention while ensuring stability of the P retained, which ultimately result in continuous net P retention at acceptable levels. Another strategy to prolong the lifetime of edge-of-field technologies consists on constructing a sedimentation basin prior to the system, especially when the load of particles and associated P is large[17]. This way, system saturation with P is expected to slow down.
A major constraint in the implementation of edge-of-field technologies refers to the use of agricultural land which would otherwise be used for cropping systems. This is especially the case for systems aiming to increase the HRT for P retention (e.g., constructed and restored wetlands), and even more if the P loads are dominated by dissolved P. Restored wetlands may require larger areas so as to achieve a more stable hydrological regime and consistent P retention, owing to the potential release of P from the P-enriched soils[41]. Thus, it is recommended to verify the level of P saturation of the soil and ascertain whether it contains sufficient amounts of P sorbents available for sorption prior to wetland restoration, in order to ensure proper performance and minimize the required area. The integrated buffer zone mentioned above was effective in the retention of P, although its area represented only 0.1% of the catchment area[20]. This indicates that this system can be an alternative in catchments with limited area available for the treatment of drainage discharge.
Another limitation of edge-of-field technologies is that these systems are generally not effective in the retention of both particulate and dissolved P forms–i.e., constructed and restored wetlands, and vegetated buffer strips are normally consistent sinks for particulate P, but show variable retention for dissolved P, while filter materials are designed to reduce dissolved P loads. However, although a few field studies demonstrated the potential of filter materials in the reduction of P loads[13][42], most experiments were conducted in the laboratory. Therefore, it is recommended additional tests under field conditions with locally available materials to ensure their successful application. The reduction of both P and nitrogen loads, on the other hand, has been tested through a combination of woodchip bioreactors with filter materials[43][44][45]. The results show that the paired systems can be rather successful in the retention of P, with cases of almost complete retention[44]. The arrangement of the paired system, however, must be taken into account to ensure proper retention. It is found that filter materials placed downstream the woodchip bioreactors show better results, as this arrangement prevents the export of P from the paired system if marked reducing conditions arise in the bioreactors with subsequent release of iron-bound P. Overly reduced effluents from the bioreactors–possibly owing to long HRTs–on the other hand, may inhibit P sorption and/or favor P desorption in the downstream filter material, hindering P retention[43]. This is a particular situation where an opposite arrangement may be preferable.
Finally, cost-efficiency assessments are crucial to determine whether the implementation of an edge-of-field technology in a specific agricultural catchment is feasible or not. A general finding is that increasing P loads lead to higher P retention and better cost-efficiency[46]. This indicates that the feasibility depends to some extent on the P loads, and likely increases in critical source areas. In the case of filters, the choice of the material has a major impact, in which locally available materials rich in iron and aluminum may represent a cost-efficient option in catchments with unstable hydrological regime.
Final remarks
Edge-of-field technologies are an alternative to agricultural catchments where in-farm management of P is not able to minimize P loss to tile drains at acceptable levels. Thus, these have been used to reduce the excess P loads from drainage discharge prior to reaching downstream surface waters, preventing eutrophication. The successful application of these measures largely depends on properly designing the systems according to local catchment conditions. For that, a P retention goal must first be defined and subsequently achieved, e.g., by determining the minimum HRT of the system; by finding the optimal width and configuration of vegetated buffer strips; and by choosing a suitable filter material. In this context, simulation and modelling of P retention performance in different systems and under varying catchment conditions represent potential methods to formulate a basis for system optimization and improve the cost-efficiency.
Implementation of edge-of-field technologies is recommended in critical source areas not only to reduce the excess P loads but also because these can be more cost-efficient when receiving higher P loads[46]. A limitation of these systems, however, is that effective retention of both particulate and dissolved P forms is generally not achieved in single systems. Therefore, pairing systems can be a promising alternative. This has been observed in systems containing a sedimentation basin as the first treatment stage, where most of the incoming particulate P settles on the upper sediments[17]. Other options may include pairing the system with a filter material in case the fraction of dissolved P from the P load is significant and the HRT is normally short to allow proper retention.
Finally, the successful application of edge-of-field technologies depends not only on consistent net P retention but also on long-term stability of the P retained. Therefore, it is essential to ensure the availability of P sorption sites so that P saturation occurs at a slow rate, in addition to the prevalence of oxidizing conditions. This could be partly achieved with regular maintenance, contributing to prolong the system lifetime and potentially improving the cost-efficiency.
This entry is adapted from the peer-reviewed paper 10.3390/app10020634
References
- B. Ulén; M. Bechmann; J. Fölster; Helen Jarvie; H. Tunney; Agriculture as a phosphorus source for eutrophication in the north-west European countries, Norway, Sweden, United Kingdom and Ireland: a review. Soil Use and Management 2007, 23, 5-15, 10.1111/j.1475-2743.2007.00115.x.
- Robert H. Foy; J. Thomas Sims; Andrew N. Sharpley; The Return of the Phosphorus Paradigm: Agricultural Phosphorus and Eutrophication. American Society of Agronomy Monograph 2005, No. 46, 911-939, 10.2134/agronmonogr46.c28.
- Lipe R. D. Mendes. Surface-flow constructed wetlands retaining phosphorus from agricultural drainage water: hydrological and biogeochemical factors controlling the retention of phosphorus and its stability in soil/sediments; Aarhus University, Department of Agroecology: Tjele, Denmark, 2018; pp. 144.
- K.W. King; Mark Williams; Merrin Macrae; Norman R. Fausey; Jane Frankenberger; Douglas R. Smith; Peter Kleinman; Larry C. Brown; Phosphorus Transport in Agricultural Subsurface Drainage: A Review. Journal of Environmental Quality 2015, 44, 467-485, 10.2134/jeq2014.04.0163.
- Riccardo Scalenghe; A. C. Edwards; F. Ajmone; E. Barberis; The effect of reducing conditions on the solubility of phosphorus in a diverse range of European agricultural soils. European Journal of Soil Science 2002, 53, 439-447, 10.1046/j.1365-2389.2002.00462.x.
- Suzanne Beauchemin; R. R. Simard; D. Cluis; Forms and Concentration of Phosphorus in Drainage Water of Twenty-Seven Tile-Drained Soils. Journal of Environmental Quality 1998, 27, 721-728, 10.2134/jeq1998.00472425002700030033x.
- M. Stone; A. Mudroch; The effect of particle size, chemistry and mineralogy of river sediments on phosphate adsorption. Environmental Technology Letters 1989, 10, 501-510, 10.1080/09593338909384766.
- Andrew N. Sharpley; Richard McDowell; Peter Kleinman; Phosphorus loss from land to water: integrating agricultural and environmental management. Plant and Soil 2001, 237, 287-307, 10.1023/a:1013335814593.
- Lars Bergström; Holger Kirchmann; Faruk Djodjic; Katarina Kyllmar; Barbro Ulén; Jian Liu; Helena Andersson; Helena Aronsson; Gunnar Börjesson; Pia Kynkäänniemi; et al. Turnover and Losses of Phosphorus in Swedish Agricultural Soils: Long-Term Changes, Leaching Trends, and Mitigation Measures. Journal of Environmental Quality 2015, 44, 512-523, 10.2134/jeq2014.04.0165.
- J D Gaynor; W. I. Findlay; Soil and Phosphorus Loss from Conservation and Conventional Tillage in Corn Production. Journal of Environmental Quality 1995, 24, 734-741, 10.2134/jeq1995.00472425002400040026x.
- SERA-17: Innovative Solutions to Minimize Phosphorus Losses from Agriculture . SERA-17. Retrieved 2020-2-26
- Peter Kleinman; Douglas R. Smith; Carl H. Bolster; Zachary M. Easton; Phosphorus Fate, Management, and Modeling in Artificially Drained Systems. Journal of Environmental Quality 2015, 44, 460-466, 10.2134/jeq2015.02.0090.
- Richard McDowell; Andrew N. Sharpley; W. Bourke; Treatment of Drainage Water with Industrial By-Products to Prevent Phosphorus Loss from Tile-Drained Land. Journal of Environmental Quality 2008, 37, 1575-1582, 10.2134/jeq2007.0454.
- Faruk Djodjic; L. Bergstrom; B. Ulén; Phosphorus losses from a structured clay soil in relation to tillage practices. Soil Use and Management 2002, 18, 79-83, 10.1111/j.1475-2743.2002.tb00223.x.
- B. Ulén; Episodic precipitation and discharge events and their influence on losses of phosphorus and nitrogen from tile-drained arable fields. Swedish Journal of Agricultural Research 1995, 25, 25-31, .
- A.T. O'geen; R. Budd; J. Gan; J.J. Maynard; Sanjai Parikh; R.A. Dahlgren; Mitigating Nonpoint Source Pollution in Agriculture with Constructed and Restored Wetlands. Advances in Agronomy 2010, 108, 1-76, 10.1016/s0065-2113(10)08001-6.
- Lipe Renato Dantas Mendes; Karin Tonderski; Charlotte Kjaergaard; Phosphorus accumulation and stability in sediments of surface-flow constructed wetlands. Geoderma 2018, 331, 109-120, 10.1016/j.geoderma.2018.06.015.
- Ed J. Dunne; N. Culleton; G. O’Donovan; R. Harrington; K. Daly; Phosphorus retention and sorption by constructed wetland soils in Southeast Ireland. Water Research 2005, 39, 4355-4362, 10.1016/j.watres.2005.09.007.
- H. K. Pant; K.R. Reddy; F. E. Dierberg; Bioavailability of Organic Phosphorus in a Submerged Aquatic Vegetation-Dominated Treatment Wetland. Journal of Environmental Quality 2002, 31, 1748-1756, 10.2134/jeq2002.1748.
- D. Zak; Brian Kronvang; Mette Vodder Carstensen; Carl Christian Hoffmann; Ane Kjeldgaard; Soren Erik Larsen; Joachim Audet; Sara Egemose; Charlotte Adam Jørgensen; Peter Feuerbach; et al. Nitrogen and Phosphorus Removal from Agricultural Runoff in Integrated Buffer Zones. Environmental Science & Technology 2018, 52, 6508-6517, 10.1021/acs.est.8b01036.
- Karin M. Johannesson; Jonas L. Andersson; Karin S. Tonderski; Efficiency of a constructed wetland for retention of sediment-associated phosphorus. Hydrobiologia 2011, 674, 179-190, 10.1007/s10750-011-0728-y.
- Pia Kynkäänniemi; Barbro Ulén; Gunnar Torstensson; Karin S. Tonderski; Phosphorus Retention in a Newly Constructed Wetland Receiving Agricultural Tile Drainage Water. Journal of Environmental Quality 2013, 42, 596-605, 10.2134/jeq2012.0266.
- Lipe Renato Dantas Mendes; Karin Tonderski; Bo Vangsø Iversen; Charlotte Kjaergaard; Phosphorus retention in surface-flow constructed wetlands targeting agricultural drainage water. Ecological Engineering 2018, 120, 94-103, 10.1016/j.ecoleng.2018.05.022.
- David A. Kovacic; Mark B. David; Lowell E. Gentry; Karen M. Starks; Richard A. Cooke; Effectiveness of Constructed Wetlands in Reducing Nitrogen and Phosphorus Export from Agricultural Tile Drainage. Journal of Environmental Quality 2000, 29, 1262-1274, 10.2134/jeq2000.00472425002900040033x.
- Chris C. Tanner; James P. S. Sukias; Multiyear Nutrient Removal Performance of Three Constructed Wetlands Intercepting Tile Drain Flows from Grazed Pastures. Journal of Environmental Quality 2011, 40, 620-633, 10.2134/jeq2009.0470.
- Magnus Land; Wilhelm Granéli; Anders Grimvall; Carl Christian Hoffmann; William Mitsch; Karin S. Tonderski; Jos T. A. Verhoeven; How effective are created or restored freshwater wetlands for nitrogen and phosphorus removal? A systematic review. Environmental Evidence 2016, 5, 1-26, 10.1186/s13750-016-0060-0.
- B.C. Braskerud; Factors affecting phosphorus retention in small constructed wetlands treating agricultural non-point source pollution. Ecological Engineering 2002, 19, 41-61, 10.1016/s0925-8574(02)00014-9.
- Moshe Shenker; S. Seitelbach; S. Brand; A. Haim; M. I. Litaor; Redox reactions and phosphorus release in re-flooded soils of an altered wetland. European Journal of Soil Science 2005, 56, 515-525, 10.1111/j.1365-2389.2004.00692.x.
- Carl Christian Hoffmann; Charlotte Kjaergaard; Jaana Uusi-Kämppä; Hans Christian Bruun Hansen; Brian Kronvang; Phosphorus Retention in Riparian Buffers: Review of Their Efficiency. Journal of Environmental Quality 2009, 38, 1942-1955, 10.2134/jeq2008.0087.
- Lewis L. Osborne; David A. Kovacic; Riparian vegetated buffer strips in water-quality restoration and stream management. Freshwater Biology 1993, 29, 243-258, 10.1111/j.1365-2427.1993.tb00761.x.
- M. Brian C. Hickey; Bruce Doran; A Review of the Efficiency of Buffer Strips for the Maintenance and Enhancement of Riparian Ecosystems. Water Quality Research Journal 2004, 39, 311-317, 10.2166/wqrj.2004.042.
- Chad Penn; Joshua McGrath; James Bowen; Stuart Wilson; Phosphorus removal structures: A management option for legacy phosphorus. Journal of Soil and Water Conservation 2014, 69, 51A-56A, 10.2489/jswc.69.2.51a.
- C. J. Penn; R. B. Bryant; P. J. A. Kleinman; A. L. Allen; Removing dissolved phosphorus from drainage ditch water with phosphorus sorbing materials. Journal of Soil and Water Conservation 2007, 62, 269-276, .
- Gry Lyngsie; O.K. Borggaard; Hans Christian Bruun Hansen; A three-step test of phosphate sorption efficiency of potential agricultural drainage filter materials. Water Research 2014, 51, 256-265, 10.1016/j.watres.2013.10.061.
- Eriona Canga; Goswin Johann Heckrath; Charlotte Kjaergaard; Agricultural Drainage Filters. II. Phosphorus Retention and Release at Different Flow Rates. Water, Air, & Soil Pollution 2016, 227, 276-288, 10.1007/s11270-016-2963-3.
- Gry Lyngsie; Chad J. Penn; Hans Christian Bruun Hansen; Ole K. Borggaard; Phosphate sorption by three potential filter materials as assessed by isothermal titration calorimetry. Journal of Environmental Management 2014, 143, 26-33, 10.1016/j.jenvman.2014.04.010.
- Chad Penn; Isis Chagas; Aleksandar Klimeski; Gry Lyngsie; A Review of Phosphorus Removal Structures: How to Assess and Compare Their Performance. Water 2017, 9, 583, 10.3390/w9080583.
- Dustin Stoner; C. J. Penn; Joshua McGrath; Jason G. Warren; Phosphorus Removal with By-Products in a Flow-Through Setting. Journal of Environmental Quality 2012, 41, 654-663, 10.2134/jeq2011.0049.
- Tsung-Min Su; Sheng-Chi Yang; Shang-Shu Shih; Hong-Yuan Lee; Optimal design for hydraulic efficiency performance of free-water-surface constructed wetlands. Ecological Engineering 2009, 35, 1200-1207, 10.1016/j.ecoleng.2009.03.024.
- S. Wenger. A review of the scientific literature on riparian buffer width, extent and vegetation; Office of Public Service and Outreach, Institute of Ecology, University of Georgia: Athens, Georgia, 1999; pp. 59.
- C. J. Woltemade; Ability of restored wetlands to reduce nitrogen and phosphorus concentrations in agricultural drainage water. Journal of Soil and Water Conservation 2000, 55, 303-309, .
- Teija Kirkkala; Anne-Mari Ventelä; Marjo Tarvainen; Fosfilt filters in an agricultural catchment: a long-term field-scale. Agricultural and Food Science 2012, 21, 237-246, 10.23986/afsci.6830.
- Laura E. Christianson; Christine Lepine; Philip Sibrell; Chad Penn; Steven T. Summerfelt; Denitrifying woodchip bioreactor and phosphorus filter pairing to minimize pollution swapping. Water Research 2017, 121, 129-139, 10.1016/j.watres.2017.05.026.
- Gregory E. Goodwin; Rabin Bhattarai; Richard Cooke; Synergism in nitrate and orthophosphate removal in subsurface bioreactors. Ecological Engineering 2015, 84, 559-568, 10.1016/j.ecoleng.2015.09.051.
- Guanghui Hua; Morgan W. Salo; Christopher G. Schmit; Christopher H. Hay; Nitrate and phosphate removal from agricultural subsurface drainage using laboratory woodchip bioreactors and recycled steel byproduct filters. Water Research 2016, 102, 180-189, 10.1016/j.watres.2016.06.022.
- Florence Gachango; S. M. Pedersen; C. Kjaergaard; Cost-Effectiveness Analysis of Surface Flow Constructed Wetlands (SFCW) for Nutrient Reduction in Drainage Discharge from Agricultural Fields in Denmark. Environmental Management 2015, 56, 1478-1486, 10.1007/s00267-015-0585-y.
