1. Supported Catalysts
Compared to non-supported catalysts, supported catalysts have obvious advantages, such as high dispersion of active components and low amount. Among multifarious catalysts reported for CO-SCR, supported Cu-based catalysts are the most extensively studied and have considerable application potential [
29]. In this section, various types of supported Cu-based catalysts and their catalytic behavior in NO reduction by CO in the presence of O
2 are summarized.
Table 1 gives the catalytic performances of supported Cu-based catalysts for NO + CO reaction in the presence of O
2 reported in the literature.
1.1. Mono-Metallic Catalysts
The loading state of the metal, the nature of the support, and the interaction between the metal and the support will all affect the catalytic performance. Yamamoto et al. [
30] studied the effects of supported elements, supports, and calcination temperature on the catalytic performance of NO-CO-O
2 reaction. The Cu-based catalysts with different supports and loading amounts were investigated in detail, and the results suggest that 0.5 wt.% Cu/Al
2O
3 exhibited the highest catalytic performance. The support γ-Al
2O
3 itself can reduce NO to N
2O but not N
2, possessing a limited capability for the reduction of NO. Therefore, the addition of Cu to Al
2O
3 promotes the formation of N
2. Additionally, the calcination temperature of the catalyst and Cu loading could affect the catalytic activity. When the calcination temperature was too high (>500 °C), the Cu species on the surface could slowly form aggregated Cu species, which preferentially oxidize CO without reducing NO
x, thereby resulting in a sharp decrease in catalytic performance. Moreover, when the Cu loading was 3 wt.%, the polymer-like Cu species were mainly present on the surface of the support. If the Cu loading was further increased to more than 5 wt.%, CuO species appeared on the catalyst surface. Both the polymer-like Cu species and CuO species mainly facilitate CO oxidation. With the 0.5 wt.% Cu loading, there was the generation of atomically dispersed Cu
2+ species on γ-Al
2O
3. In this case, the oxidation activity of CO was weak, and a large amount of residual CO could interact with NO. Thus, the reduction activity of NO by CO is the highest. Nevertheless, Sierra-Pereira et al. [
26] found that for CuO/TiO
2, its activity increased with Cu loading from 2 wt.% to 10 wt.% in NO-CO-O
2 reaction, and 10 wt.% CuO/TiO
2 exhibited the highest catalytic performance, achieving 54% NO conversion at 500 °C.
In addition to the above single metal oxide support, metal oxide composite supports were also applied to optimize the denitration performance of Cu-based catalysts. AlPO
4, which has two different types of surface hydroxyl groups, (AlOH) and (POH), is a kind of stable material with large specific surface area and acid properties [
47]. Kacimi et al. [
31] prepared a series of Cu/AlPO
4 catalysts with different Cu loadings by Cu(II) ion complexes exchange, which leads to the formation of well-dispersed Cu(II) amino species. Among these catalysts, 5Cu/AlPO
4, containing the largest amount of dispersed surface Cu(II) species, exhibited the best catalytic performance, achieving 90% NO conversion at 300 °C. Venegas et al. [
32] reported that the Cu/SmCeO
2@TiO
2 catalyst with Cu supported on core–shell-structured SmCeO
2@TiO
2 achieved 50% NO conversion at 500 °C in the presence of 10 vol.% O
2. Its superior catalytic performance was because CeO
2 possessed excellent redox properties through the transfer between Ce
3+ and Ce
4+, thus increasing the oxidation activity of the Cu/SmCeO
2@TiO
2 catalyst. Moreover, the addition of Sm helped to maintain the thermal stability of the CeO
2 phase. Core–shell-structured CeO
2@TiO
2 nanoparticles could also stabilize the involved Cu phase, preventing its migration and sinterization, and thus leading to higher activities [
48]. Bai et al. [
33] synthesized an efficient CuO/CeO
2-Al
2O
3 catalyst, which exhibited excellent catalytic performance and superior resistance to O
2 and SO
2 for CO-SCR. The incorporation of Ce
4+ was conducive to the enrichment of Cu atoms and the generation of synergistic oxygen vacancies on the surface of the catalyst, which improved the redox performance of the catalyst. Moreover, Cu
2+ was favorable for the CO adsorption, while the unpaired electrons in the CeO
2-Al
2O
3 support were favorable for the adsorption of NO.
1.2. Bimetallic Catalysts
Chen et al. [
43] synthesized a series of CuCoO
x/TiO
2 catalysts and found that the CuCoO
x/TiO
2 catalyst able to generate the CuCo
2O
4 spinel exhibited the highest catalytic activity, reaching 98.9% NO conversion at 200 °C and in the absence of O
2. However, when 2 vol.% O
2 was introduced, the NO conversion decreased sharply to 60%. Liu et al. [
42] investigated the denitration performance of various transition metals supported on Al
2O
3 pellets under O
2-rich conditions (16 vol.%). Among these catalysts, Cu-Mn/Al
2O
3 with a molar ratio Cu:Mn of 1.5 displayed the best catalytic activity, achieving nearly 78% NO conversion and 85% N
2 selectivity at 180 °C. Based on the density functional theory calculation, it was demonstrated that Mn had better O
2 resistance and Cu had better H
2O resistance. López et al. [
41] prepared a novel core–shell-structured K/Cu/SmCe@TiO
2 catalyst, giving 97% NO conversion at 330 °C in the presence of excess O
2 (10 vol.%). The interaction between highly dispersed Cu species and K promoted the reduction of NO. Gholami et al. [
40] found that the catalytic activity of the Cu1:Ce3/CNT catalyst (carbon nanotubes) was much better than that of the Cu1:Ce3/AC catalyst (activated carbon) in the presence of O
2 (0.3 vol.%, O
2/CO ≥ 0.6). The Cu1:Ce3/CNT catalyst displayed the highest NO conversion of 96% at 220 °C, attributed to its high concentration of surface oxygen vacancies (SOVs), high Cu
+ species content, superior reducing capability, and the synergistic effect between SOV and Cu
+ species. Furthermore, Gholami et al. [
39] investigated the denitration performance of a string of Cu1:Ce3 catalysts supported on various supports (CNTs, AC, TiO
2, γ-Al
2O
3, and SiC) in the presence of excess O
2 (5 vol.%), and found that Cu1:Ce3/Al
2O
3 catalyst possessed the highest catalytic performance, with 71.8% NO conversion at 420 °C, mainly ascribed to the enrichment of catalytically active centers of Cu on the Al
2O
3 support. Interestingly, it was observed that with the increase in O
2 concentration from 2% to 5%, the conversion of NO increased slightly. This was because the more O
2 was adsorbed on the catalyst surface, the more adsorbed O was provided. The adsorbed O then reacted with the adsorbed CO to form CO
2, which thus led to the generation of oxygen vacancies for the adsorption and dissociation of NO further. Moreover, this adsorbed O could also react with NO to NO
2, which was quickly reduced by CO to N
2. Metal organic frameworks (MOFs) have broad application prospects in the field of catalysis, due to their huge surface area, tailored compositions, and variable structures [
49,
50]. Zhang et al. [
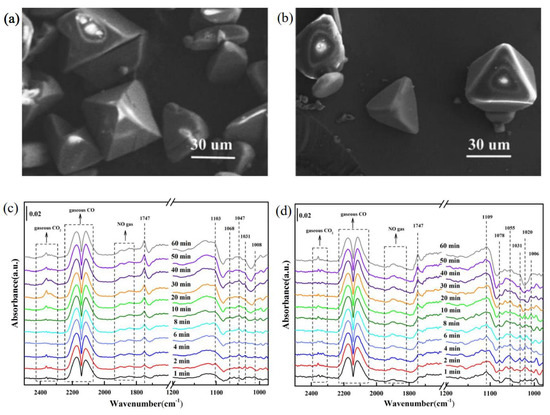
37] prepared the Cu-BTC (BTC = benzene-1,3,5-tricarboxylate) and Ce-Cu-BTC catalysts, which are three-dimensional (3D) porous MOFs (their SEM images are shown in
Figure 1a,b). Cu-BTC only exhibited 50% NO conversion at 250 °C, while Ce-modified Cu-BTC catalysts could achieve much higher NO conversion of 91%. Owing to the incorporation of Ce
3+, the Ce-Cu-BTC catalyst had more SOVs, conducive to enhancing the adsorption of NO
x on the surface of catalysts, as evidenced by the in situ DRIFTS spectrum (
Figure 1c,d). The enhanced NO
x adsorption ultimately improved the catalytic activity for CO-SCR.
Figure 1. SEM images of (
a) Cu-BTC and (
b) Ce-Cu-BTC. In situ DRIFTS spectra of CO + NO + O
2 co-adsorption on the surface of (
c) Cu-BTC and (
d) Ce-Cu-BTC at 150 °C, where NO = 1000 ppm, CO = 1000 ppm, 5 vol.% O
2, and equilibrium gas was N
2. Reproduced from [
37] with permission.
Recently, researcher's group used a simple impregnation method followed by reduction with H2 to synthesize a Pt-Cu@M-Y catalyst, which consists of sub-nanometric Pt on Cu nanoparticles confined in the NaOH-modified Y-zeolite. The Pt-Cu@M-Y catalyst with only 0.04 wt.% Pt loading showed superior catalytic activity for NO + CO reaction, with NO conversion and N2 selectivity nearly 100% at 250 °C. This enhanced activity originated from the synergistic catalysis of Pt and Cu, in which NO was mainly adsorbed on sub-nanometric Pt, and the generated interfaces between Cu nanoparticles and surface CuOx species served as the dissociation sites of NO. However, when 1 vol.% O2 was introduced (O2/CO = 10), the NO conversion decreased to 43% and N2 selectivity dropped to 53% at 350 °C, due to the preferential oxidation of CO and NO by O2 at high temperatures.
1.3. Multi-Metallic Catalysts
Pan et al. [
46] synthesized a series of Cu-based and Mn-based catalysts by the wet impregnation method and applied them to the CO-SCR reaction. It was found that Cu-Ce-Fe-Co/TiO
2 and Mn-Ce-Fe-Co/TiO
2 exhibited better catalytic activity in the absence of O
2, both reaching full NO conversion at 250 °C. However, the presence of O
2 largely restricts the NO reduction efficiency. Comparatively, Cu-Ce-Fe-Co/TiO
2 showed better tolerance to O
2 than Mn-Ce-Fe-Co/TiO
2. When 6 vol % O
2 was fed, the Cu-Ce-Fe-Co/TiO
2 catalyst still exhibited 93% NO conversion and 74.3% NO
x conversion at 200 °C ([NO] = 200 ppm, [CO] = 200 ppm), indicating that only a part of NO was oxidized. The enhanced catalytic performance of Cu-Ce-Fe-Co/TiO
2 may owe to its superior reducibility, more oxygen vacancies, and better oxygen mobility. Wang et al. [
45] synthesized a Cu-Ni-Ce/AC catalyst by the ultrasonic equal volume impregnation method. This catalyst exhibited extremely high catalytic activity in the presence of O
2 (5 vol.%), reaching 99.8% NO conversion at 150 °C. In this case, the doping of Ce promoted the uniform dispersion of Cu and Ni and formed many reaction units on the surface of the catalyst, enhancing the adsorption abilities of CO and NO and thus improving the catalytic performance. Two-dimensional (2D) vermiculite (VMT) is a natural layered clay mineral with a unique two-dimensional structure and high-temperature stability, widely used as a support and applied in the fields of photocatalysis and heterogeneous catalysis. Liu et al. [
44] synthesized a CuCoCe/2D-VMT catalyst by the impregnation method. It exhibited superior catalytic activity in the coexistence of 1 vol.% O
2 and 5 vol.% H
2O, reaching 70% NO conversion and 97% N
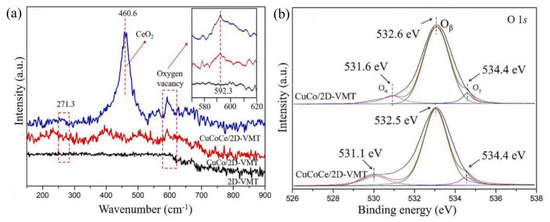
2 selectivity at 200 °C. They found that the doping of Ce could reduce the reduction temperature and promote the formation of oxygen vacancies, giving the CuCoCe/2D-VMT sample more active centers, thus improving its catalytic performance. This deduction was confirmed by the Raman spectrum (
Figure 2a), in which the CuCoCe/2D-VMT sample had a higher concentration of oxygen vacancies than the CuCo/2D-VMT sample. Similar results were also obtained by XPS characterization. As shown in
Figure 2b, CuCoCe/2D-VMT had more adsorbed oxygen (denoted as O
β).
Figure 2. (
a) Raman spectra of CuCoCe/2D-VMT, CuCo/2D-VMT, and 2D-VMT catalysts and (
b) XPS spectra of O 1
s. Reproduced from [
44] with permission.
2. Non-Supported Catalysts
Besides a large number of reported Cu-based supported catalysts, some non-supported Cu-based catalysts also have certain O
2 resistance in the CO-SCR reaction. Mehandjiev et al. [
51] first reported that CuCo
2O
4 had the ability to reduce NO by CO in the presence of O
2. Furthermore, Panayotov et al. [
52] found that Cu
xCo
3−xO
4 spinels possessed excellent catalytic performance for CO-SCR under O
2-containing conditions than CuO and Co
3O
4. Additionally, in the presence of 650 ppm O
2, the catalytic activity increased with the Cu content. Ivanka et al. [
53] prepared CuO-MnO
x (1.5 < x < 2) catalysts by coprecipitation and studied their catalytic performance in the presence of O
2. They found that the degree of NO conversion to N
2 achieved by CuO-MnO
x (Cu/Cu + Mn up to 0.53) under O
2-containing conditions was similar to that under O
2-free conditions. This could be explained as follows. After the introduction of O
2, NO quickly reacted with it to produce NO
2. Moreover, the reduction of NO
2 by CO was faster than CO oxidation. Therefore, N
2 and CO
2 were finally generated. Sun et al. [
54] synthesized the CuCe mixed metal oxides, which showed superior NO conversion and N
2 selectivity, both maintaining more than 90% in a wide temperature window in the absence of O
2. Nevertheless, when 1% O
2 was introduced, the NO conversion dropped rapidly to 0 within 3.5 h. The NO conversion could gradually recover to the initial value after the O
2 was stopped. This result indicated that CO preferentially reacts with O
2, resulting in the decrease in NO conversion in the presence of O
2. Wen et al. [
55] synthesized mixed CuCeMgAlO oxides by coprecipitation, which possessed higher NO conversion than CuMgAlO and CeMgAlO for NO + CO + O
2 reaction. The superior catalytic performance of CuCeMgAlO can be explained by the synergistic effect generated by the interaction of Cu and Ce. In addition, when 1% H
2O was introduced, the NO conversion over CuCeMgAlO was significantly improved from 50% to 100% at 250 °C, but both CuMgAlO and CeMgAlO lost their catalytic activity completely. Moreover, when 500 ppm SO
2 was introduced, the NO conversion dropped rapidly over CuMgAlO and CeMgAlO; however, CuCeMgAlO still maintained 100% NO conversion at 720 °C. This suggests that CuCeMgAlO possesses high activity for NO + CO + O
2 reaction and excellent resistance to H
2O and SO
2 poisoning.
This entry is adapted from the peer-reviewed paper 10.3390/catal12111402