Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The spatially heterogeneous distribution of soil nutrients is ubiquitous in terrestrial ecosystems and has been shown to promote the performance of plant communities, influence species coexistence, and alter ecosystem nutrient dynamics. Plants interact with diverse soil microbial communities that lead to an interdependent relationship (e.g., symbioses), driving plant community productivity, belowground biodiversity, and soil functioning.
- arbuscular mycorrhizal fungi (AMF)
- clonal plants
- foraging mechanism
1. Introduction
In terrestrial ecosystems, plants interact with a myriad of soil microbial communities that lead to the establishment of interdependent relationships [1], which drive plant community productivity [2], belowground biodiversity, and ecosystem multifunctionality [3][4][5]. These interactions are crucial for many aspects [6][7], including the nutrient acquisition of plants from heterogeneously distributed microsites. The responses of plants to spatially, heterogeneously distribute soil nutrients require a specialized physiological strategy commonly referred to as the root foraging mechanism, i.e., the proliferation of roots in nutrient-rich microhabitats [8][9] and microbially mediated mechanisms via a plant–microbe symbiotic relationship to ensure the effective acquisition of soil nutrients [10]. For example, arbuscular mycorrhizal fungi (AMF) are essential to these plant–soil microbe interactions [11]. Whilst both soil microorganisms and soil nutrient heterogeneity are known to influence plant performance, community productivity, and competitive interactions [2], little is known about the potential impacts of soil microorganisms on the effect of soil nutrient heterogeneity on plant growth (see [12][13]).
Soil nutrient heterogeneity may be defined as the variability in the distribution of the available soil nutrients within the soil matrix in a given microhabitat [14][15]. The unequal distribution of available nutrient resources may originate from various sources, such as uneven distribution and decomposition of litter in the soil [16][17], differences in the parent material during weathering processes, topography, climate, and differences in the availability of microorganisms [14][15]. Spatial heterogeneity of soil nutrients is ubiquitous in the ecosystem and plays a critical role in the growth of the individual plant [13][18][19], population structure [20], community productivity [21][22], intraspecific and interspecific interaction, and species coexistence [19][23][24][25]. One mechanism underlying such an impact in plants is the capacity of the root foraging response by the roots in nutrient-rich microsites [9][26]. However, the size or scale of the heterogeneity (i.e., patch scale) [27] and the differences in nutrient level (i.e., patch contrast) are the two major characteristics of soil nutrient heterogeneity that can influence plant growth [28]. A common reason is that the responses of plants to soil nutrient heterogeneity via the foraging mechanism are highly dependent on both patch scale and patch contrast [29]. Previous studies have shown that a plant can respond to soil nutrient heterogeneity at one scale but may be unresponsive at another [8][30][31]. Therefore, the patch size and contrast of soil nutrients can determine the effect of spatial heterogeneity on plant growth [32][33]. Nevertheless, the activities of soil microorganisms may influence the distribution of soil nutrients irrespective of the patch size or patch contrast [13][34], thereby altering the effect of soil nutrient heterogeneity on plant performance.
The soil microbial communities constitute a diverse group of microorganisms whose activities can positively or negatively impact the growth and productivity of plants [2][6]. In effect, soil microorganisms directly influence plant growth by forming a mutual (symbiotic) or pathogenic relationship with the roots and, through the free-living microorganisms (non-symbiotic) that are indirectly capable of switching the rate of nutrient supply to plants [2]. Common among these root-associated microorganisms are AMF, which have the potential to supply limiting nutrients such as soil phosphorus (P) to its host in return for carbon [35]. This AM fungal-plant association is the most ancient and abundant relationship in the terrestrial ecosystem [36][37]. Moreover, the N-fixing bacteria provide the highest quantity of soil N for plant community productivity in most ecosystems, especially in plant communities dominated by legumes [2][38]. This N represents about 20% of the total N needed by plants annually [39][40]. Indirectly, there is a considerable number of microorganisms that are N-fixers but are not in a direct symbiotic relationship with any vascular plant, e.g., free-living N2 fixers, cyanobacteria that fix N2 via its symbiotic association with lichens and bryophytes [39]. Additionally, some filamentous actinomycetes, such as Frankia, are known to fix N2 as free-living or when in a symbiotic relationship with nonluminous vascular plants [39][41]. Despite these pieces of accumulating evidence that soil microorganisms can influence the growth of plants, knowledge of how these belowground communities mediate plant nutrient acquisition in patchily distributed soil nutrients in the terrestrial ecosystem is still limited.
2. Ecological Importance of Soil Nutrient Heterogeneity
It is well known that the pattern of distribution in soils of essential nutrients for plant growth always displays variation at a range of different spatial scales. Over the years, significant ecological investigations on soil nutrient heterogeneity have been carried out on individual plant growth [19][21], population and community productivity [20][42], and ecosystem nutrient dynamics [43]. Across all findings, evidence is accumulating that the most significant influence of biodiversity on the terrestrial ecosystem functioning is dependent on the heterogeneously distributed soil nutrient [43]. The main reasons underlying such species’ response to the spatial distribution of soil nutrients include enhancing competitive, interspecific interactions in plant communities. As soil nutrients are ubiquitous in space and time, competition for available soil nutrients may depend on plant functional group identity and diversity [43][44]. For example, Garcia Palacios et al. found species-specific responses of three functional groups (grasses, legumes and non-legumes forbs and a combination of them) to heterogeneously distributed soil nutrients in terms of plant biomass accumulation.
Moreover, plants have varying foraging strategies for nutrient uptake in a heterogeneous environment. These strategies include the selective placement of roots into high-nutrient patches, changes in biomass allocation [23][25][45], and the modification of nutrient uptake capacity [46]. Such differences in the belowground nutrient acquisition are likely to induce disparities in growth and biomass accumulation, thus resulting in the exclusion of the slow-growing plants from the ecosystem by the fast-growing plants [23]. In this way, soil nutrient heterogeneity can modulate the composition and structure of plant communities.
Additionally, the patch scale and patch contrast are inherent features of soil nutrient heterogeneity [21][30][47]. Therefore, plant responses to unevenly distributed soil nutrients may rely on species’ sensitivity to a specific patch scale [48]. Thus, another species may be successful in a multi-species environment if one species fails to acquire nutrients from a specific patch scale. It is worth noting that patch contrast (i.e., the level of nutrient availability) may influence nutrient acquisition and plant demand for a particular nutrient type that is likely to limit its growth [49]. Moreover, the level of nutrient availability, in some cases, compels some plant species with little nutrient demand to employ avoidance mechanisms [20] while others aggressively proliferate their roots in this environment [50]. Such complementary responses of individual species to heterogeneously distributed nutrients may promote species coexistence and the efficient use of ecosystem nutrients.
Lastly, soil nutrient heterogeneity modulates ecosystems’ response to global environmental change. The global-change drivers interacting with soil nutrient heterogeneity include elevated nitrogen deposition, altered rainfall patterns, and increased atmospheric CO2 [51]. The beneficial effects of soil nutrient heterogeneity on plant performance in species and population studies mainly occur under a high-nutrient availability [26][27][29]. For example, Maestre and Reynold [52] observed a significant increase in the shoot biomass of an experimental grassland community owing to the interaction between soil nutrient heterogeneity and nutrient availability. Likewise, it is reported that soil nutrient heterogeneity regulated the impact of elevated atmospheric CO2 on grassland nutrient use efficiency [42]. One underlying mechanism expected to stimulate such interaction within the plant community level is the presence of co-occurring species, which may lead to differences in plant responses to soil nutrient heterogeneity, suggesting that the plant-nutrient uptake capacity in a heterogeneous environment will likely determine soil heterogeneity as a modulator of ecosystem responses to a surge in atmospheric CO2.
3. Ecological Impacts of Soil Microorganisms
The activity of soil microbial communities is considered the lifeline of global ecosystem productivity and sustainability [53][54]. However, plant response to the functional diversity of these unseen soil communities is a crucial determinant of the structure and composition of plant communities. The essential roles that are very critical to the functioning of both above- and belowground plant productivity include, but are not limited to, molecular transformation (organic matter transformation and inorganic transformation), nutrient cycling (nitrogen fixation and carbon cycling), and soil transformation (formation and development of soil).
3.1. Molecular Transformation (Organic Matter and Inorganic Transformation)
Plants require inorganic N for growth [55][56][57]; however, a greater proportion (c. 95%) of nitrogen (N) in terrestrial ecosystem soil is in an organic form as amines and amides [58]. Therefore, access to this inorganic N requires a complete decomposition and mobilization of these molecules [55]. Plants depend on soil microorganisms for the enzymatic liberation of soil N and make it available for use [59]. The mycorrhizal fungi are an essential group of soil microorganisms that enhances soil N acquisition by plants. AMF and ectomycorrhizal fungi (ECM) are two significant players in plant–mycorrhizal interaction in the ecosystem [60][61]. These microorganisms are involved in the assimilation of amino acids and sugars [58] and the degradation of proteins [7], thereby making the embodied N available to their host. For example, saprophytic fungi and some soil bacteria can equally immobilize soil N from organic materials; however, ECM can use lignocellulolytic enzymes to break down the organic molecule and release the inorganic N to the host [58]. Mycorrhizal fungi, therefore, modify plant inorganic soil N uptake by increasing the absorbing surface area and the volume of soil exploited by the fine root system of plants [58]. Such soil microbial-assisted plant inorganic N acquisition is vital for deciphering the soil biogeochemical cycles and ecosystem sustainability.
3.2. Nutrient Cycling (Nitrogen Fixation and Carbon Cycling)
Nutrient availability determines the plant communities’ above- and belowground productivity, ecosystem stability, and nutrient dynamics [54][62][63]. As a result of intermittent, essential nutrient (e.g., N and P) deficiencies and limitations in natural systems [64], plant–microorganism interaction becomes necessary to offset such nutrient challenges [1][65][66]. Thus, soil microorganisms supply their host with the requisite N and P in return for soil carbon [37]. One such plant–soil microbe interaction is the leguminous–rhizobia interaction that replenishes N naturally into the soil (referred to as biological nitrogen fixation) [67].
Previous studies have indicated that nitrogen fixation accounts for about 97% of soil N [68][69][70]. Other microbial partners (e.g., Burkholderia and Cupriavidus) can nodulate legumes and fix soil N [71][72]. Besides the well-known legume-rhizobia, other free-living microorganisms nodulate with a diverse group of angiosperms to fix N, also referred to as asymbiotic nitrogen fixation [69]. Recent evidence suggests that asymbiotic nitrogen fixation constitutes a crucial N input to terrestrial ecosystems with limited symbiotic partners [73].
3.3. Soil Formation
The formation and fertility of the soil from parent materials involves physical, chemical, and biological processes [74][75]. The properties of the soil formed are influenced by factors like topography, time, climate, and parent material, as well as the plants and soil microorganisms present [75]. However, the nutrients available for plant growth highly depend on the microorganism’s type, functional identity, and microbial biomass. Therefore, the types, functional identity, and microbial biomass play a vital role in nutrient transformation, nutrient storage, and nutrient cycling [76], which can be a reliable indicator of the soil quality, the stability of the belowground food web, and the ecosystem functioning. For example, in an earlier study, Taunton et al. [76] observed that the soil microbial communities were responsible for regulating phosphate and lanthanide distribution during weathering and soil formation.
4. Plants—Soil Microorganisms—Soil Nutrient Heterogeneity Loop
Plant ecologists have extensively considered the interaction between plants and their microbiomes in the last few decades. These studies span the individual species of plants [77][78] and soil microorganisms with a recent consideration of populations and community studies [60]. The soil microbial communities play critical roles in plant growth, which has a strong implication for ecosystem development and nutrient dynamics [58][59]. Similarly, plants exert some form of reciprocal effects on the function and biodiversity of the soil community. Most of the benefits derived from soil microorganisms and plants directly and indirectly affect growth and productivity. For example, accumulated evidence from previous studies has indicated that through the photosynthetic processes, plants supply the required C to the soil community in exchange for the most limiting nutrients [37]. Indeed, the exudates from plants and litter distribution are not uniformly arranged within the soil matrix, resulting in various scales of heterogeneity and a varying contrast of the soil nutrients.
Such spatial heterogeneity of soil nutrients has been extensively studied and has been found to promote plant growth, species composition, and coexistence with plant communities [28][29]. It is known that soil nutrient heterogeneity mediates intra-and inter-specific competitive interaction with neighboring plants [25]. However, the responses of plants to the effects of spatial heterogeneity of soil nutrients are most often species specific. A simple reason is that the scale and contrast of soil nutrients can influence soil heterogeneity [21][30], which determines the extent of sensitivity or the responses of plants to soil nutrient heterogeneity [48]. As a result, plants have developed various strategies and mechanisms to effectively uptake available soil nutrients.
However, the influence of soil microorganisms on plant responses to spatial heterogeneity of soil nutrients has not gained much attention in the scientific community. Therefore, more research studies are needed to bridge the gap and to increase the scope of understanding the potential roles of soil microorganisms on nutrient uptake, particularly under conditions of patchily distributed habitats. Soil nutrient availability is essential for plant growth and the belowground food web. The interactions of these three key factors (plant, soil microorganisms, and soil nutrient heterogeneity) converge to enhance ecosystem productivity (Figure 1).
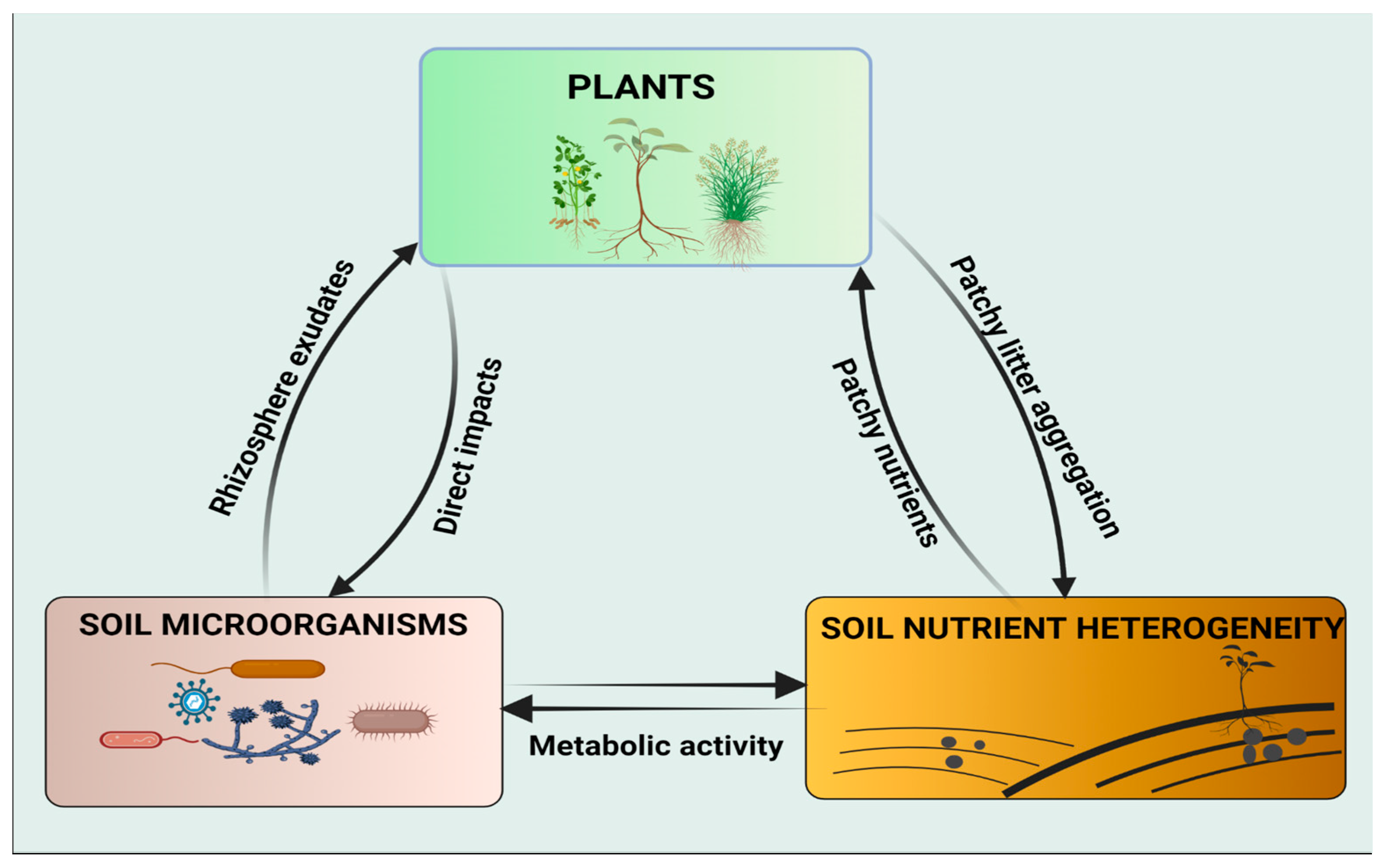
Figure 1. Schematic representation of the interactions between the plant, soil microorganisms, and soil nutrient heterogeneity. Both plants and soil microorganisms can acquire their nutrients from patchy microsites and alter soil properties by organic litter decomposition and metabolic activities, respectively. Soil microorganisms have diverse, direct effects on plants, e.g., mineralization of organic matter and homogenization of patchy nutrients. Plants interact with soil microorganisms through metabolites exuded by the roots, particularly in the rhizospheric zone.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10122399
References
- Mitter, B.; Pfaffenbichler, N.; Sessitsch, A. Plant–microbe partnerships in 2020. Microb. Biotechnol. 2016, 9, 635–640.
- van der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310.
- Harris, J. Soil microbial communities and restoration ecology: Facilitators or followers? Science 2009, 325, 573–574.
- Johnson, D.; IJdo, M.; Genney, D.R.; Anderson, I.C.; Alexander, I.J. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi? J. Exp. Bot. 2005, 56, 1751–1760.
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270.
- Chandra, P. Soil–microbes–plants: Interactions and ecological diversity. In Plant Microbe Interface; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2019.
- Read, D.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492.
- Cahill, J.F.; McNickle, G.G.; Haag, J.J.; Lamb, E.G.; Nyanumba, S.M.; St. Clair, C.C. Plants integrate information about nutrients and neighbors. Science 2010, 328, 1657.
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24.
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233.
- Kawaguchi, M.; Minamisawa, K. Plant–microbe communications for symbiosis. Plant Cell Physiol. 2010, 51, 1377–1380.
- Du, J.; Yu, F.-H.; Alpert, P.; Dong, M. Arbuscular mycorrhizal fungi reduce effects of physiological integration in Trifolium repens. Ann. Bot. 2009, 104, 335–344.
- Adomako, M.O.; Xue, W.; Du, D.-L.; Yu, F.-H. Soil biota and soil substrates influence responses of the rhizomatous clonal grass Leymus chinensis to nutrient heterogeneity. Plant Soil 2021, 465, 19–29.
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2005, 57, 401–411.
- Stark, J. Causes of soil nutrient heterogeneity at different scales. In Exploitation of Environmental Heterogeneity by Plants; Caldwell, M., Pearcy, R., Eds.; Academic Press: San Diego, CA, USA, 1994; pp. 255–284.
- Cai, A.; Chang, N.; Zhang, W.; Liang, G.; Zhang, X.; Hou, E.; Jiang, L.; Chen, X.; Xu, M.; Luo, Y. The spatial patterns of litter turnover time in Chinese terrestrial ecosystems. Eur. J. Soil Sci. 2019, 71, 856–867.
- Loydi, A.; Lohse, K.; Otte, A.; Donath, T.W.; Eckstein, R.L. Distribution and effects of tree leaf litter on vegetation composition and biomass in a forest–grassland ecotone. J. Plant Ecol. 2013, 7, 264–275.
- Dong, B.-C.; Alpert, P.; Zhang, Q.; Yu, F.-H. Clonal integration in homogeneous environments increases performance in Alternanthera philoxeroides. Oecologia 2015, 179, 393–400.
- Zhou, J.; Dong, B.C.; Alpert, P.; Li, H.-L.; Zhang, M.-X.; Lei, G.-C.; Yu, F.-H. Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides. Ann. Bot. 2012, 109, 813–818.
- Day, K.J.; Hutchings, M.J.; John, E.A. The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. J. Ecol. 2003, 91, 305–315.
- Birch, C.P.D.; Hutchings, M.J. Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. J. Ecol. 1994, 82, 653–664.
- Adomako, M.O.; Gao, F.-L.; Li, J.-M.; Du, D.-L.; Xue, W.; Yu, F.-H. Effects of soil nutrient heterogeneity and parasitic plant infection on an experimental grassland community. Flora 2020, 271, 151666.
- Xue, W.; Huang, L.; Yu, F.-H.; Bezemer, T.M. Intraspecific aggregation and soil heterogeneity: Competitive interactions of two clonal plants with contrasting spatial architecture. Plant Soil 2018, 425, 231–240.
- de Kroon, H.; Hendriks, M.; van Ruijven, J.; Ravenek, J.; Padilla, F.M.; Jongejans, E.; Visser, E.J.W.; Mommer, L. Root responses to nutrients and soil biota: Drivers of species coexistence and ecosystem productivity. J. Ecol. 2012, 100, 6–15.
- Zhang, L.-M.; Alpert, P.; Yu, F.-H. Nutrient foraging ability promotes intraspecific competitiveness in the clonal plant Hydrocotyle vulgaris. Ecol. Indic. 2022, 138, 108862.
- Hutchings, M.J.; de Kroon, H.D. Foraging in plants: The role of morphological plasticity in resource acquisition. Adv. Ecol. Res. 1994, 25, 159–238.
- Jackson, R.B.; Caldwell, M.M. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 1993, 74, 612–614.
- Wang, P.; Alpert, P.; Yu, F.-H. Clonal integration increases relative competitive ability in an invasive aquatic plant. Am. J. Bot. 2016, 103, 2079–2086.
- Hutchings, M.; Wijesinghe, D. Performance of a clonal species in patchy environments: Effects of environmental context on yield at local and whole-plant scales. Evol. Ecol. 2008, 22, 313–324.
- Bliss, K.M.; Jones, R.H.; Mitchell, R.J.; Mou, P.P. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytol. 2002, 154, 409–417.
- Chen, D.; Ali, A.; Yong, X.H.; Lin, C.G.; Niu, X.H.; Cai, A.M.; Dong, B.C.; Zhou, Z.X.; Wang, Y.J.; Yu, F.H. A multi-species comparison of selective placement patterns of ramets in invasive alien and native clonal plants to light, soil nutrient and water heterogeneity. Sci. Total Environ. 2019, 657, 1568–1577.
- Fransen, B.; de Kroon, H.; Berendse, F. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 1998, 115, 351–358.
- Qian, Y.-Q.; Luo, D.; Gong, G.; Han, L.; Ju, G.-S.; Sun, Z.-Y. Effects of spatial scale of soil heterogeneity on the growth of a clonal plant producing both spreading and clumping ramets. J. Plant Growth Regul. 2014, 33, 214–221.
- Reynolds, H.L.; Haubensak, K.A. Soil fertility, heterogeneity, and microbes: Towards an integrated understanding of grassland structure and dynamics. Appl. Veg. Sci. 2009, 12, 33–44.
- Georgiou, K.; Abramoff, R.Z.; Harte, J.; Riley, W.J.; Torn, M.S. Microbial community-level regulation explains soil carbon responses to long-term litter manipulations. Nat. Commun. 2017, 8, 1223.
- Hodge, A.; Robinson, D.; Fitter, A.H. An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient-rich patches in soil. New Phytol. 2000, 145, 575–584.
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647.
- Pajares, S.; Bohannan, B.J. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016, 7, 1045.
- Cleveland, C.C.; Townsend, A.R.; Schimel, D.S.; Fisher, H.; Howarth, R.W.; Hedin, L.O.; Perakis, S.S.; Latty, E.F.; Von Fischer, J.C.; Elseroad, A.; et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Glob. Biogeochem. Cycles 1999, 13, 623–645.
- Van Der Heijden, M.G.A.; Streitwolf-Engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Ineichen, K.; Boller, T.; Wiemken, A.; Sanders, I.R. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 2006, 172, 739–752.
- Benson, D.R.; Silvester, W.B. Biology of Frankia strains, actinomycete symbionts of actinorhizal plants. Microbiol. Rev. 1993, 57, 293–319.
- Maestre, F.T.; Reynolds, J.F. Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Glob. Chang. Biol. 2006, 12, 2431–2441.
- García-Palacios, P.; Maestre, F.T.; Gallardo, A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. J. Ecol. 2011, 99, 551–562.
- Alpert, P.; Holzapfel, C.; Slominski, C. Differences in performance between genotypes of Fragaria chiloensis with different degrees of resource sharing. J. Ecol. 2003, 91, 27–35.
- Weiser, M.; Koubek, T.; Herben, T. Root foraging performance and life-history traits. Front. Plant Sci. 2016, 7, 1–7.
- Liu, L.; Alpert, P.; Dong, B.-C.; Yu, F.-H. Modification by earthworms of effects of soil heterogeneity and root foraging in eight species of grass. Sci. Total Environ. 2020, 708, 134941.
- Campbell, B.D.; Grime, J.P.; Mackey, J.M.L. A trade-off between scale and precision in resource foraging. Oecologia 1991, 87, 532–538.
- Wijesinghe, D.; Hutchings, M. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: An experimental study with Glechoma hederacea. J. Ecol. 1997, 85, 17–28.
- Lamb, E.G.; Haag, J.J.; Cahill Jr, J.F. Patch–background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti. Funct. Ecol. 2004, 18, 836–843.
- Gersani, M.; Brown, J.s.; O’Brien, E.E.; Maina, G.M.; Abramsky, Z. Tragedy of the commons as a result of root competition. J. Ecol. 2001, 89, 660–669.
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214.
- Maestre, F.; Roynolds, J. Amount or pattern? Grassland responses to the hetergeneity and availability of two key resources. Ecology 2007, 88, 501–511.
- Wagg, C.; Jansa, J.; Schmid, B.; van der Heijden, M.G. Belowground biodiversity effects of plant symbionts support aboveground productivity. Ecol. Lett. 2011, 14, 1001–1009.
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262.
- Schimel, J.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602.
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823.
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry 2014, 118, 471–486.
- Pellitier, P.T.; Zak, D.R. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: Why evolutionary history matters. New Phytol. 2018, 217, 68–73.
- Nicolás, C.; Martin-Bertelsen, T.; Floudas, D.; Bentzer, J.; Smits, M.; Johansson, T.; Troein, C.; Persson, P.; Tunlid, A. The soil organic matter decomposition mechanisms in ectomycorrhizal fungi are tuned for liberating soil organic nitrogen. ISME J. 2019, 13, 977–988.
- Hannula, S.E.; Träger, S. Soil fungal guilds as important drivers of the plant richness–productivity relationship. New Phytol. 2020, 226, 947–949.
- Zhang, Z.; Yuan, Y.; Liu, Q.; Yin, H. Plant nitrogen acquisition from inorganic and organic sources via root and mycelia pathways in ectomycorrhizal alpine forests. Soil Biol. Biochem. 2019, 136, 107517.
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283.
- Matías, L.; Castro, J.; Zamora, R. Soil-nutrient availability under a global-change scenario in a Mediterranean mountain ecosystem. Glob. Chang. Biol. 2011, 17, 1646–1657.
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379.
- Liang, Y.; Pan, F.; Jiang, Z.; Li, Q.; Pu, J.; Liu, K. Accumulation in nutrient acquisition strategies of arbuscular mycorrhizal fungi and plant roots in poor and heterogeneous soils of karst shrub ecosystems. BMC Plant Biol. 2022, 22, 188.
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal fungi—Potential organic matter decomposers, yet not saprotrophs. New Phytol. 2015, 205, 1443–1447.
- Bano, S.; Iqbal, S.M. Biological nitrogen fixation to improve plant growth and productivity. Int. J. Agric. Innov. Res. 2016, 4, 597–599.
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892.
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45.
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226.
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.M.; Elliott, G.N.; Bontemps, C.; Estrada-de Los Santos, P.; Gross, E.; Dos Reis, F.B.; Sprent, J.I.; et al. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant Microbe Interact. 2011, 24, 1276–1288.
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767.
- Reed, S.C.; Vitousek, P.M.; Cleveland, C.C. Are patterns in nutrient limitation belowground consistent with those aboveground: Results from a 4 million year chronosequence. Biogeochemistry 2011, 106, 323–336.
- Semenov, A.M.; Đukić, D.A. The role of microbial communities in soil formation and soil ecosystem health. Paleontol. J. 2020, 54, 843–852.
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996.
- Taunton, A.E.; Welch, S.A.; Banfield, J.F. Microbial controls on phosphate and lanthanide distributions during granite weathering and soil formation. Chem. Geol. 2000, 169, 371–382.
- Adomako, M.O.; Xue, W.; Du, D.-L.; Yu, F.-H. Soil microbe-mediated N:P stoichiometric effects on Solidago canadensis performance depend on nutrient levels. Microb. Ecol. 2021, 83, 960–970.
- Adomako, M.O.; Xue, W.; Tang, M.; Du, D.-L.; Yu, F.-H. Synergistic effects of soil microbes on Solidago canadensis depend on water and nutrient availability. Microb. Ecol. 2020, 80, 837–845.
This entry is offline, you can click here to edit this entry!
