Cancer is one of the longest-known human diseases, known at least from ancient Egyptian papyri. Even though the causal association between cancer and occupational exposure to pollutants can be inferred from the works of Paracelsus, only in the early 20th century onward, when the nature and role of DNA was unravelled, could oncobiologists and toxicologists join efforts to endeavour understanding mechanism and risk. Nowadays we know that chemically-induced cancers of environmental origin (excluding tobacco smoking) can represent about 10% or more of the total number of incidences, globally. The paradigmatic case of asbestos in the second half of the 20th century was arguably the first incident to increase the awareness for environmental carcinogens in a global scale. However, it resulted in a long and painstaking ban process that altogether highlights the challenges of safeguarding human and environmental health.
1. Introduction
Cancer is one of the longest-known human diseases. The first scientific descriptions are attributed to the Greek Hippocrates, the ‘Father’ of Medicine (≈460–370 BC), to whom the analogy between malignancies and ‘crab’ (with which a Greek wording for cancer,
carcinos, is related), due to the projection-like outgrowths of the disease resembling the decapod crustaceans’ locomotory appendages. The Greek physician Galen (130–200 AD), practicing in Rome, first used the term
oncos (‘growth’) to describe cancer, which stands today as the prefix of cancer-related terms such as oncogenesis (the complex process by which normal cells transform into cancer cells). Nonetheless, the oldest medical mentions to cancer date back to at least 1600 BC, even though they are likely based on information collected up to a thousand years before, if not more. They were found in ancient Egyptian writing (the ‘Edwin Smith’ and ‘George Ebers’ papyri) that, inclusively, already mentioned treatment by surgery, pharmacology and magic rituals [
1,
2].
Today, cancer is acknowledged as the principal cause of mortality worldwide, being responsible for about 10 million fatalities annually (with about twice as many new cases per year), figures that, led by in cases and death rate by lung cancer, can only partly be explained by population growth or socioeconomical development, as they may double in the next decade [
3]. Even though the exact proportion of chemical-induced cancer is extremely difficult to estimate, Colditz and Wei [
4] estimated that, excluding tobacco smoking, it may attain 10%. The same authors also noted that, due to legislation reducing occupational exposure for workers of high-risk industries (such as steel mills) in developed countries from late 20th century onwards, risk can actually be underestimated globally, since a great deal of heavy industry has been transferred to less-developed countries, where cases are likely underreported. Altogether, the incidence of cancer driven or at least co-adjuvated by exposure to pollutants represents a very significant portion of all cases and is seemingly increasing. As suggested by the European Commission Joint Research Centre (EC-JRC), “
Public health policy actions cannot be decoupled from environmental policy actions, since exposure to chemicals through air, soil, water and food can contribute to cancer and other chronic diseases” (sic Madia et al. [
5]). Needless to say, the challenges of describing and mitigating human health risks are but a fraction of those related to ecosystem and wildlife. Considering that malignancies have been found in 70-million-year-old dinosaur fossils [
6], it is safe to assume that cancer is transversal at least among vertebrates, which renders environmental carcinogens not just a key problem for environmental toxicologists in general, but also to ecotoxicologists.
Cancer is essentially a genetic disease. Despite its long record in human medicine, the causes of cancer only began to be understood in the 20th century, especially after the discovery of the role of DNA and its chemistry; first in 1944 with the discovery by Avery et al. [
7] that DNA is the ‘transforming principle’ in heredity; then with the famous ‘photo 51′ of Franklin and Gosling [
8], which was paramount to the double helix of Watson and Crick [
9]. Interestingly, the first human oncogene was only described in the early 1980s [
10], following the pioneer work of Duesberg and Vogt [
11] on avian retroviruses and transforming factors leading to sarcomas (malignancies of mesodermal-origin tissues, such as sub-dermal connective tissue). Almost immediately, Reddy et al. [
12] disclosed molecular events underneath proto-oncogene activation into full oncogene, disclosing that all it may take is a single-point mutation. Quite naturally, this raised important questions among genotoxicologists, as it provided important insights on the mechanisms by which pollutants are oncogenic, even though we know today that different toxicants imply distinct modes-of-action, an issue that will be addressed further on. In the aftermath of these discoveries, just before the end of the 20th century, toxicologists and oncobiologists discovered that certain toxicants can cause specific mutations to oncogenes, leading to up-regulation of oncoproteins and therefore unbalancing cell cycle in favour of proliferation with prejudice of DNA checkpoints, cell cycle arrest and apoptosis; or, conversely, hindering the expression of tumour suppressors such as TP53. One of the most significant discoveries relates to polycyclic aromatic hydrocarbons (PAHs), many of which yield mutagenic metabolites (such as epoxides and some quinones) following phase I bioactivation by CYPs [
13]. We now know that the formation of bulky DNA-metabolite adducts is not stochastic. Instead, there are preferential binding sites in specific regions of proto-oncogenes, such as those of the human ras family, promoting mutation due to problems in the removal of these lesions that lead to the conversion of the genes in oncogenes. increasing expression and subsequent up-regulation of oncoproteins (see Ross and Nesnow [
14]). Considering the prevalence of PAHs in emissions such as tobacco smoke plus fumes and their particulate matter, this issue has been receiving specific attention due to its implications in human lung cancer (reviewed by Moorthy et al. [
15], DeMarini and Linak [
16]). The paradigmatic case study of PAH mutagenicity and carcinogenicity illustrates that cell and molecular biology laid important foundations for establishing causation and both human and ecological risk assessment. However, there are other fields of research that revolutionised toxicology and must thus not be forgotten. It is the case of the teachings of the British mathematician turned biologist Ronald Fisher (1890–1962), which, despite being involved mostly in population genetics (he was a polemic advocate of eugenics and endorsed human race distinction), arguably constructed the basis of modern statistics and biostatistics that are paramount for toxicologists and that are in the core of computational biology methods. Similar acknowledgements must be given to epidemiology, environmental chemistry and ecology.
It must be noted that pollutant-induced carcinogenesis in humans is just a small part of a large environmental problem. Even though the nature and function of detoxification phase I/II enzymatic systems and their role in neoplasia in non-vertebrate animals is far from consensual, in large part due to unstable substrate-dependent evolution from common ancestry [
17,
18], they tend to be well-conserved amongst vertebrates, including fish, which sustains that chemical mutagenesis and oncogenesis are also a serious ecotoxicological problem. In fact, ecotoxicologists have been in the frontline of research and are responsible for major developments in risk assessment, biomarker plus bioassay development and toxicogenomics. This contribution owes much to the fact that ecotoxicologists have to deal with challenges like toxicant mixtures in intricate matrices (such as natural sediments); unconventional model species with reduced or absent genomic annotation and unaccountable noise variables compromising causation [
13,
19,
20].
2. A Brief Historical Overview of Cancer and Pollution
The great scientific gap of the Dark Ages brought biomedical sciences in western civilisation to a stand-still. However, Arabic physicians like Avicenna (980–1037) and philosophers like Averroes (1126–1198) collected, expanded and taught scientific knowledge, essentially keeping alive the flame of early biomedicine in times of strict Christian fundamentalism and feudal obscurantism. Their work and teachings were fundamental pillars upon which European medicine would be rebuilt during the Renaissance. The Swiss-German physician and alchemist Theophrast von Hohenheim (1493–1541), better known as Paracelsus, who is considered the founder of modern toxicology by first acknowledging dose-effect relationships (‘it is only the dose which separates benefit from poison’), was seemingly the first to draw conclusions on chemical-induced carcinogenesis. In his posthumously published work on the ‘mountain disease’ of miners (1567), Paracelsus already linked illnesses such as tuberculosis and lung cancer to exposure to ‘poisonous air’. This work (originally named
Von der Bergsucht und anderen Bergkrankheiten) was born from Paracelsus’ direct observations of miners and their environment. It can very well be the first occurrence of what we would nowadays call an association between occupational exposure and cancer—in a time were diseases were mostly attributed to mystical causes (the reader is diverted to the review by Hayes and Gilbert [
21] on the hallmarks of toxicology). Additionally, the Italian physician Bernardino Ramazzini (1633–1714), often regarded to be the founder of occupational medicine, in his most famous book,
De Morbis Artificum Diatriba (‘On the Diseases of Workers’), already discussed the environment-related aetiology of various diseases, from pneumoconiosis to breast cancer (see Franco [
22]). It needs to be noted, though, that if Theophrast established a link between toxicology and disease and Ramazzini associated occupational exposure to disease, including cancer, the study of chemical toxicology at this stage was still struggling with establishing causation due to the lack of identification of carcinogens
per se. Nonetheless, despite rudimentary or
a priori absent tools, The Illuminist Man would slowly, but steadily, find his course (see
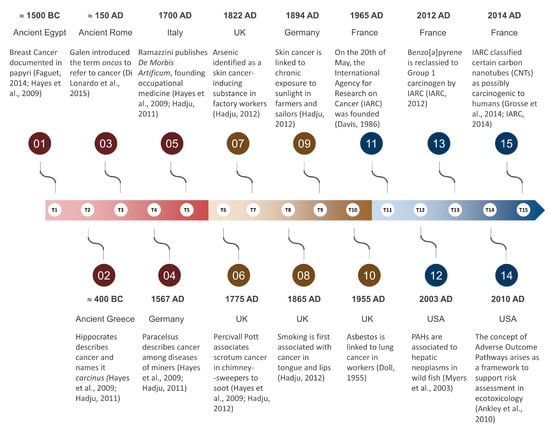
Figure 1 for a historical overview).
Figure 1. Important milestones in ‘oncotoxicology’, from the first written records to modern days. Main sources [
2,
19,
21,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32] are indicated as per reference list.
In the times that preceded the Industrial Revolution, the English physician Percivall Pott (1714–1788), building on the work of Paracelsus, associated testicular squamous cell carcinoma, a typical scrotum malignancy with chimney sweepers and exposure to soot. This can effectively be regarded as the first time the carcinogenicity of pyrogenic (combustion-derived) polycyclic aromatic hydrocarbons (PAHs) was documented, even though it would take about 200 years to understand the chemical nature of carcinogens in tars. In fact, only in the 1920s were PAHs identified as the carcinogenic agents in coal tars, with emphasis on the (in)famous benzo[a]pyrene [
33]. Such discoveries followed a series of experiments with murines ultimately led by the British pathologist Ernest Kennaway but based on the works on skin cancer by two outstanding Japanese pathologists, Katsusaburo Yamagiwa and his assistant Koichi Ichikawa [
34], who are effectively accredited for the discovery of environmental chemical carcinogenesis in the aftermath of their experiments on chemically induced cancer by painting tars on the ears of rabbits. These pioneering works are directly aligned with the reports on the prevalence of skin cancers among workers handling paraffins, petroleum and tars dating from the previous century [
35,
36]. These examples illustrate the difficulties in establishing causation even well after the First Industrial Revolution (late 1700s to late 1800s). In large part, this is due to the lack of a well-defined experimental method (including statistics) in life sciences and the missing gaps in what we call today biology 101 that made it impossible for researchers to understand
why cancer occurs, which is a paramount question that still puzzles present-day scientists. In fact, despite of the discovery of cells by Robert Hooke (1635–1703), published in 1665 in his book ‘
Micrographia’, only in the mid-19th century was Cell Theory effectively established as one of the dogmas of Biology by the hands of three German scientists, Theodor Schwann, Matthias Jakob Schleiden and Rudolf Virchow, who, nevertheless, worked autonomously. The 19th century also witnessed the revelation of evolution and natural selection through Darwin, Wallace and Haeckel; Mendelian heredity and DNA itself in 1869 (as ‘nuclein’) by the Swiss chemist Friedrich Miescher (1844–1895). It would require a century before these three hallmarks of biology would be pieced together in a unified theory, though. This means that the mechanistic perception of cancer as a genetic disease with origin in mutations, hereditary or acquired, would have to wait more than 100 years to mature. Nonetheless, in 1866, the eminent French physician Pierre Broca (1824–1880) had already noted hereditary predisposition for breast cancer in some families. Additionally, the Nobel prize-winning US pathologist Francis Peyton Rous (1979–1970) identified for the first time that a pathogen (a retrovirus infecting chickens) could cause cancer (the Rous Sarcoma that would later be fundamental for the abovementioned discovery of oncogenes).
Also of great importance is the link between the formation of micronuclei and neoplasic cells by the German cytologist Theodor Boveri (1862–1915) from his work with echinoderm embryos [
37]. Micronuclei and other nuclear abnormalities are, to date, acknowledged biomarkers of malignancies and of genotoxicity (damage at whole-chromosome level, in the case), spanning from humans to wildlife [
38,
39,
40]. Together with the alkaline Comet assay method for the detection DNA lesions at the strand levels [
41], the quantification of nuclear abnormalities in peripheral cells (including the haemocytes of invertebrates) is still one of the most important and expeditious biomarkers for genotoxicants deployed by environmental toxicologists and ecotoxicologists. The first-time micronuclei were associated with exposure to an environmental agent (ionising radiation, in the case) occurred in 1959 [
42]. Interestingly, this happened well after the discovery of X-ray-induced DNA damage and mutagenesis in
Drosophila in the 1920s [
43]. It also followed the anecdotal case of the wealthy US golf amateur champion and socialite Eben Byers (1880–1932), who had a gruesome death after being devoured by mouth and gut cancer (not radiation poisoning as reported at the time), after years of drinking radioactive water, a patented tonic (‘Radithor’) that was advertised as an endocrine stimulator by the famous charlatan William Bayley in a time when selling radioactive pseudo-medicines, cosmetics and materials was common practice, without neglecting the early amazement and overuse of X-ray medical imaging. We may also recall the sad case of the ‘radium girls’ of the 1920s, US workers that suffered from all sorts of radiation-related sickness acquired from watch dial ‘self-luminous’ radioactive paints [
44].
The end of World War II unleashed an era of technological marvel that, just like the Industrial Revolution(s) before it, was responsible for the mass production of old and novel chemicals. However, as previously mentioned, neither environmental quality not occupational medicine were mainstream issues. The status quo would change with a series of events starting in the 1960s that demystified that the environment was an infinite entity that could absorb all of our waste and dilute it to safe levels. Rachel Carson denounced pollution by pesticides, especially organochlorines like DDT (dichloro-diphenyl-trichloroethane), which was developed in the 1940s and the first modern, safe, synthetic insecticide [
45], and shortly after Clair Patterson did the same on the catastrophic global contamination by lead from fuel additives [
46]. Even though it would take decades to see any effective consequences for Carson’s and Patterson’s invaluable work, the United Nations Stockholm Convention of 2001 imposed a worldwide ban on DDT (with few emergency exceptions related to the control of insects as disease vectors), whereas Algeria was the last country in the world to stop commercialising leaded gasoline, in 2021. Importantly, it must be noted that DDT is nowadays considered by the International Agency for Research on Cancer (IARC) as ‘probably carcinogenic to humans’ (Group 2A), similarly to inorganic forms of the highly toxic metal lead (Pb), based on sufficient evidence on experimental animals, even if not from humans [
47,
48]. These case studies illustrate perfectly challenges met by scientists, particularly the fierce opposition they face when environmental and human health collide with major economic factors.
Altogether, the 1960s and 1970s formed an era of very active social and environmental activism that, regardless of the actual speed of implementation of policy and guidelines, led to profound societal changes. Besides bans and limitations on specific substances (some which will be addressed in a subsequent section), IARC itself was founded in 1965; the U.S. National Oceanic and Atmospheric Administration (NOAA) National Status and Trends (NS&T) Mussel Watch Program started in 1986 and is the oldest running marine biomonitoring programme in the world, focusing on more than 100 priority pollutants, including many environmental carcinogens; the implementation of the International Chemical Safety Cards (ICSCs) by the World Health Organisation (WHO) and the International Labour Organisation (ILO) as a tool to protect workers’ safety, or the London Convention of 1972, which aimed at prohibiting the dumping of hazardous waste in the oceans, military or civilian (signed by just 90 countries to the present day, though), to quote a few examples. Still, despite such measures, the first compelling causal relationship between PAH pollution and tumours in marine fish in their native environment was only established in the early 21st century [
29]. In another example, the potential carcinogenicity of certain multi-walled carbon nanotubes (MWCNTs) was only acknowledged by IARC [
31,
32] just 23 years after the unravelling of CNTs [
49], hitherto motivated by the growing interest in nanotechnology. These two instances illustrate not only the rapid advance of toxicological sciences but also awareness for cancer-inducing agents. Nonetheless, it is clear that there is still a considerable time gap between the introduction of a new chemical (or nanomaterial) and acknowledging its risks, which is yet another challenge that must urgently be resolved. In fact, twenty-three years may be seen as short period, but it effectively represents the span of a generation. And it took only the time of a generation to witness the rapid expansion of quack radioactive pseudo-medicines and cosmetics between 1920s and the 1950s. Even though our current level of knowledge and, moreover, risk assessment policies and guidelines (which emerged from the synergy between science and public awareness more than from goodwill of political or economic leadership) greatly diminishes the possibility of these events being repeated, they are not unlikely to happen given the impressive variety and quantity of new compounds that are introduced in the market yearly. The combined effect of legacy (traditional) and novel (emerging) pollutants on humans and ecosystems will be ascertained in the near future and our role in risk assessment and mitigation will be judged by future generations.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20021040