Corrosion of steel reinforcement due to chloride attack remains a major reinforced concrete durability concern. The problem is prevalent for concrete structures located within marine environments or frost-prone locations where chlorides containing de-icing salts are used.
- chloride
- sulphate
- chloride binding
- cement
- concrete
- chloride ingress
- durability
1. Introduction
Chloride-induced corrosion of steel reinforcement has been identified as one of the primary causes of early deterioration and failure of marine concrete structures, bridges, viaducts, tunnels on roads and highways where de-icing salts are applied during the winter period. Hence, one of the greatest challenges facing the construction industry is how to ensure the longevity of reinforced concrete structures. A key concern is the ingress of chlorides, which are known to cause the initiation of embedded steel bar corrosion by de-passivating the protective film provided by the high alkalinity of concrete pore solution around the steel bar [1]. Aggressive, chloride-rich environments can arise through, for example, the application of de-icing salts or the presence of aggressive marine environments. In the latter situation, the principal agents of attack are a combination of chlorides and sulphates from seawater [2][3][4].
Furthermore, changes in binder composition, with increasing use of supplementary cementitious materials and cement-replacement materials, leads to changes in phase assemblage, more structure and pore solution pH. However, these binders also offer increased aluminate levels, which can bind both sulphates and chlorides [5][6][7].
2. Resistance of Concrete to External Chlorides
Chloride attack can be considered as either internal or external. Internal sources of attack are thankfully rare, but can arise due to inappropriate admixtures, chloride-contaminated aggregates or chloride-bearing mix water. External chloride attack is more prevalent and arises through the application of de-icing salts or through the ingress of brackish water or seawater.
Exposure conditions have significant effects on the rate of chloride ingress. The requirement for water as a transport medium means that airborne chlorides pose little danger, while cyclic wetting and drying allows for the accumulation of salts through crystallization during drying periods and more severe attack[8][9][10][11]. Chloride ingress into permanently wet concrete lies somewhere between these extremes. For this reason, most national and international standards incorporate exposure classes in their specifications for concretes. For example, BS EN 206[12] sets out minimum requirements for concretes to resist chlorides, other than from seawater (XD classes) and chlorides from seawater (XS classes). Each of these are sub-divided into three, with XD/XS1 for exposure to airborne chlorides, XD/XS2 for chlorides ingressing into permanently submerged concrete and XD/XS3 for structures exposed to cyclic wetting and drying, such as tidal, splash and spray zones.
The rate at which external chlorides permeate into concrete is often used as an indicator of the concrete’s durability, with lower rates indicating more durable structures. The chloride diffusion coefficient (Dc) describes the rate of chloride ingress, and can be derived by fitting laboratory or field chloride ingress data into chloride diffusion models. Various models have been used to describe chloride ingress, as shown in Table 1. For saturated concrete, chloride ingress is assumed to be governed by pure diffusion[13], while for unsaturated concrete, e.g. for concrete exposed to alternate wetting and drying cycles, chloride ingress is governed by a combination of diffusion and convection[14][15][16].
2.1. Factors Influencing the Rate of Chloride Ingress into Concrete
The rate of chloride ingress into concrete depends on the pore structure of the hardened cement paste, which is in turn affected by many factors, such as curing conditions, w/b ratio, binder content and binder composition, including the use of supplementary cementitious materials (SCMs). These are briefly presented in Table 1, while the role of chloride binding is discussed in the next section.
Table 1. Factors influencing the rate of chloride ingress into concrete.
|
Factor |
Effect |
References |
|
Porosity |
The finer the pore structure, the greater the resistance to ingress of aggressive species, including chlorides. |
|
|
Curing conditions |
Prolonged curing reduces porosity and hence permeability, so enhances resistance to chloride ingress. Elevated temperatures, e.g., 40 °C and above, while increasing the degree of hydration, lead to a more porous network for a given degree of hydration. This leads to higher rates of chloride ingress. |
|
|
w/b ratio |
At any given temperature, higher values of w/b will result in higher rates of chloride ingress |
|
|
Binder content |
The cementitious binder provides a route by which chlorides may be bound to the hydrated cement paste. Higher binder contents lead to slower chloride ingress into the concrete |
|
|
SCMs |
SCMs such as fly ash and GGBS, when used as partial replacement materials for PC lead to reduced porosity, and hence can reduce the rate of chloride ingress |
2.2. The Role of Chloride Binding
When external chlorides permeate into concrete, they can exist as free ions dissolved in the pore water, can be bound chemically in the form of Friedel’s or Kuzel’s salt or bound physically to the surface of the hydration products (e.g., C-S-H). Since free chlorides induce reinforcement corrosion, chloride binding improves concrete durability. Also, Friedel’s salt formation can lead to pore blocking, retarding chloride ingress[1][30][31]. Hence, increased chloride binding reduces chloride migration towards the reinforcement.
2.3. Factors Affecting Chloride Binding
Several factors chloride binding, such as the cement type, alkalinity of the pore solution, cation type of the salt, chloride concentration, temperature, presence of other anions, e.g., sulphates and carbonates, and the presence of SCMs in the mix, as discussed below.
1. Cement type:
Chlorides can be readily bound as Friedel’s salt (Ca2Al(OH)6(Cl, OH)·2H2O), so higher aluminate contents encourage chloride binding. For pure cements, the C3A content is an important determinant of chloride binding capacity. Thus, sulphate-resisting Portland cements exhibit low chloride binding capacities and can lead to greater free chloride penetration for a given permeability than ordinary Portland cements[32][33].
2. Alkalinity of the pore solution:
The free chloride to hydroxide ratio is proportional to reinforcement corrosion susceptibility. However, the stability of bound chlorides also decreases with increasing pore solution pH (Figure 1), with Friedel’s salt being more soluble at pH > 12[34].
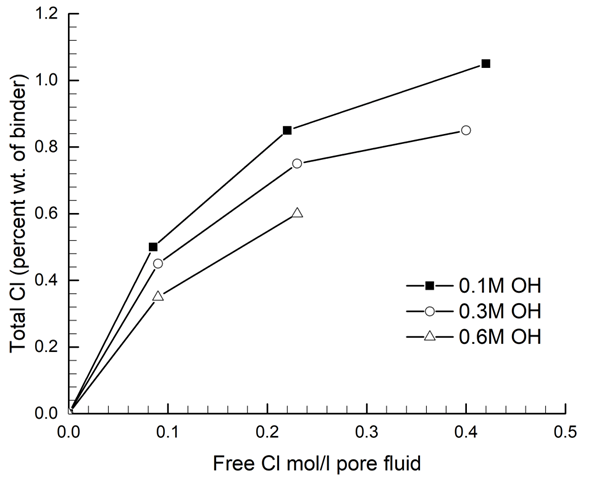
Figure 1. Effect of hydroxyl ion concentration on chloride binding for OPC pastes with water–cement ratio of 0.40 [35].
3. Cation type of the salt:
The nature of the cations associated with chlorides affects chloride binding[36][37][38], with increased binding upon exposure to calcium and magnesium chlorides than to sodium chloride[37] (see Table 2). This is due to the cation’s influence on chloroaluminate solubility and cation-induced changes in pore solution pH[34]. Sodium ions raise the pH, thus increasing Friedel’s salt solubility and reducing chloride binding.
Table 2. Influence of cation type on chloride binding for 2-day-old PC paste samples (w/c = 0.5) immersed in 20 g Cl/L solution for 28 days[37].
|
Cl Content (% by wt. of Cement) |
NaCl |
CaCl2 |
MgCl2 |
|
Free |
0.831 |
0.765 |
1.480 |
|
Bound |
0.804 |
1.408 |
2.347 |
|
Total |
1.635 |
2.173 |
3.827 |
|
Bound/Total (%) |
50 |
65 |
61 |
4. Concentration of chloride solution:
Chloride binding capacity increases with increasing chloride concentration (Figure 2) [39], due to changes in the Cl-/OH- ratio in the pore fluid.
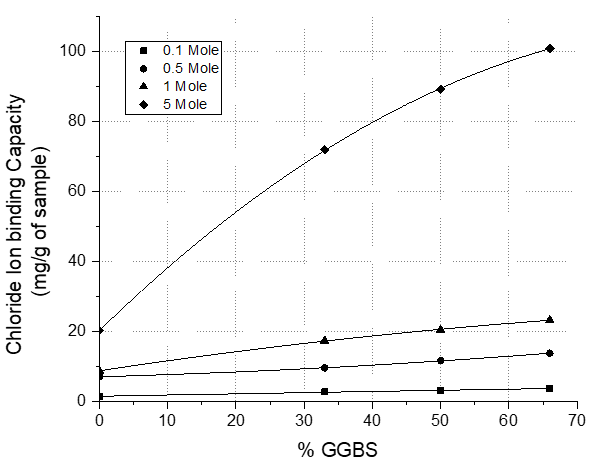
Figure 2. Effect of varying concentrations of chloride ions on chloride binding capacity[39].
5. Temperature:
The effect of temperature on chloride binding is complex, with mixed results being reported in the literature. Some studies have reported increased chloride binding with increasing temperature, while others have reported the opposite. However, in most of the latter case, the reduction in chloride binding was seen at significantly higher temperatures, i.e. circa 70oC. Increased binding has often been associated with increased degrees of cement hydration at elevated temperatures.
6. Presence of other anions:
The presence of other anions, e.g., sulphates and carbonates, may affect the chloride binding capacity of the cements. This has been attributed mainly to reactions between the various anions in the pore solution.
Reduced chloride binding in the presence of sulphates is due to the preferential reaction of C3A with sulphates[40] to form ettringite. This reduces the availability of C3A to bind chlorides as Friedel’s salt.
Meanwhile, carbonation leads to changes in phase assemblage and a reduction in pore solution pH. This reduced pH destabilizes Friedel’s salt[41][42] and reduces chloride binding.
7. Presence of SCMs:
SCMs primarily affect resistance to chloride ingress by modifying the pore structure and reducing permeability. However, SCMs also affect chloride binding, the extent of which depends on the type of SCM.
-
GGBS:
The addition of GGBS to PC increases the chloride binding capacity. This is due to: (1) GGBS having a higher aluminate content than OPC[21][27][39][40][43], (2) increased hydrotalcite formation, especially at high slag contents[44][45], (3) a typically lower sulphate content in PC-GGBS blends[40][46][47] and (4) the formation of a C-A-S-H phase responsible for binding, through adsorption, of about two-thirds of the chloride[48].
The chloride binding capacity of PC–slag blends is also dependent on the level of slag replacement, the chemical composition of the slag and the curing temperature. As with C3A contents of cements, slags with higher alumina contents increase chloride binding[21], while increased slag replacement also increases aluminate contents, so increases chloride binding[39].
-
Fly ash:
As with GGBS, the use of fly ash increases chloride binding[49][50][51]. Again due to increased alumina contents and formation of Friedel’s salt. However, at very high replacement levels, fly ash can reduce chloride binding capacity[26], due to the more gradual formation of C-S-H, which therefore reduces chloride binding by adsorption.
Chloride binding in fly ash cements can also be influenced by other factors. Prolonged curing improves chloride binding[52], due to the slow pozzolanic increasing the quantity of C-S-H present in the hardened cement paste. Curing with internal sources of chlorides can lead to increased chloride binding[37], presumably due to the accelerating effect of chlorides on cement hydration. Mixes containing class C fly ashes have been found to show improved chloride binding capacities than those containing class F ashes[53]. However, it is difficult to know if this is due to changes in phase assemblage or the increased reactivity of class C ashes leading to increased C-S-H formation and reduced permeability.
-
Metakaolin:
Metakaolin increases chloride binding, and its use is widespread in concrete exposed to chloride-rich environments. Metakaolin’s high chloride binding capacity is attributed mainly to its high alumina content.
-
Silica fume:
Unlike the other SCMs mentioned above, silica fume has a low aluminate content. Thus its impact on chloride binding is mixed. While most studies[25][36][37][54][55] report decreased chloride binding capacity, the addition of silica fume can sometimes increase chloride binding capacity[56][57]. Chloride binding can be reduced by the pozzolanic reaction between portlandite and silica fume decreasing the pore solution pH and clinker dilution reducing the C3A content. Conversely, the pozzolanic reaction leads to C-S-H formation and so increases chloride binding. Whether chloride binding increases or decreases thus depends on the replacement level. Above ca. 10% replacement chloride binding is reduced, but it is decreased at replacement levels below 5%.
-
Limestone:
While not strictly cementitious, a drive to reduce carbon footprints means that limestone is also increasingly used as a cement-replacement material. However, the use of limestone alters the hydration and microstructure of the hardened cement paste.
The replacement of up to about 15% clinker with limestone leads to pore structure refinement due to the filler effect, and so reduced permeability. This hinders chloride ingress[58]. However, higher replacement levels lead to increased clinker dilution and so to pore coarsening. Limestone in cement can react with C3A with the formation of carboaluminates. This can inhibit chloride binding in Friedel’s salt and lead to increased free chloride contents.
-
Calcined clay:
Decreasing global availability of traditional SCMs has led to the consideration of calcined clays as suitable replacements, so long as the clays show appreciable kaolinite contents. As such, binary Portland cement–calcined clay systems are expected to show performance akin to Portland cement–metakaolin blends, albeit without the significant pore refinement and improved chloride binding capacity seen for those systems. However, in recent years, low-grade calcined clays have shown significant potential in limestone calcined clay (LC3) cements.
-
Limestone Ternary Cements:
Up to 50% clinker replacement is possible without loss of performance by blending clinker with a mixture of limestone and an SCM[59]. The early-age improvement in performance induced by the addition of limestone is complemented by the subsequent reaction with aluminates in the SCMs to produce carboaluminate phases. Again, a combination of pore structure refinement and modified phase assemblages can have an impact on chloride binding[60].
Various ternary blends have been investigated, containing various SCMs (fly ash, GGS or calcined clay). Performance is influenced by the reactivity of the SCM and the replacement level. Prolonged hydration leads to reduced porosity and so reduced chloride ingress. The extent of this reduction depends on aluminosilicate hydration. Thus, improved performance is seen sooner in GGBS and calcined clay blends than in fly ash blends. The former also offer the potential for greater clinker replacement without loss in performance than fly ash blends.
In addition to changes in permeability, ternary cements also possess modified phase assemblages, which can affect chloride binding. Chloride binding increases with increasing degree of hydration and aluminate content[61]. Thus, ternary limestone cements show higher chloride binding potentials than limestone cements, but lower binding capacities than equivalent binary cements.
2.4. Chloride Binding Isotherms
Chloride binding isotherms are mathematical models relating free chlorides to bound chlorides. The simplest of these are linear, but they are an oversimplification except for very low chloride concentrations, such as in non-marine field conditions[35][62]. Consequently, non-linear isotherms are considered more appropriate, the two most common of which are the Freundlich and Langmuir isotherms. These isotherms were originally derived to describe gas adsorption on solid surfaces, where the Langmuir isotherm assumed monolayer coverage on a homogeneous surface and the Freundlich isotherm assumed multilayer coverage. In solid-solution systems, the situation is much more complicated and the underlying assumptions may no longer be valid. Therefore, the isotherms should be considered merely as empirical models and may be expressed in the forms given below:
Freundlich isotherm:
Langmiur isotherm:
where α and β are adsorption constants, which vary for different cement types. These constants do not have any physical meaning as they are not material properties but can be used to give an indication of the chloride binding capacities of the cementitious materials[21][27].
The Freundlich isotherm is more applicable at free chloride concentrations above 0.01 mol/L, while the Langmuir isotherm is more suitable for free chloride concentrations below 0.05 mol/L[63] (see Figure 3).
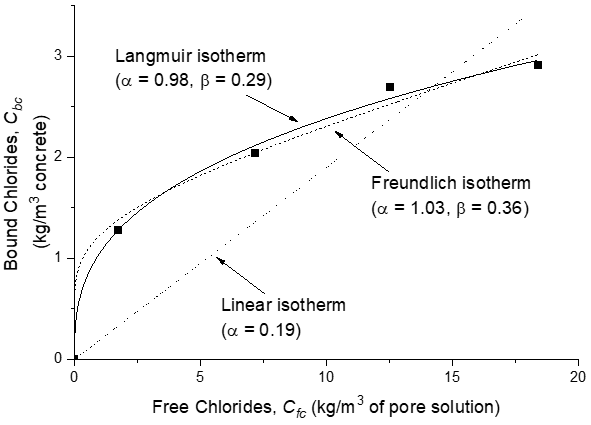
Figure 3. Plots showing linear and non-linear chloride binding isotherms[64].
3. Resistance of Concrete to External Chloride in the Presence of Sulphate
Most research has considered chloride binding in isolation from other anions. But this is unrealistic in natural environments, despite standards recognizing a difference between marine and non-marine chlorides. Of key importance is the co-existence of chlorides and sulphates, such as found in sea water.
Both chlorides and sulphates react with hydrated aluminate phases to form Friedel’s salt and ettringite, respectively. However, when present concomitantly, sulphates preferentially bind to aluminates, thus reducing chloride binding[65][66][67]. However, many factors can affect these interactions, as discussed below.
3.1. Effect of Chlorides on Sulphate Deterioration
While the general concensus is that chlorides mitigate sulphate attack, some studies have found that chlorides may accelerate sulphate attack or have no significant effect[68]. However, differences in material compositions and testing regimes between studies have made comparisons difficult.
With chlorides considered as an admixture, i.e. mixed within the cement paste, Harrison[68] found that sodium chloride did not have any substantial effect on sulphate attack, while calcium chloride increased the rate of sulphate attack.
Pure Portland cement (CEM I, Type I) systems show considerable long-term expansion upon exposure to pure sulphate solutions. This is due to the formation of ettringite. However, ettringite solubility increases in chloride solutions. Thus, sulphate-induced expansion is reduced[69] (Figure 4), but not avoided.
Composite cements, e.g. those containing GGBS, undergo reduced expansion upon exposure to sulphates. Consequently, the presence of chlorides has minimal effect on the reported expansion of composite cements[70]. The situation is slightly different when limestone cements are used, where research has been concerned with the risk of thaumasite formation. The situation depends on binder composition, plus both the chloride and sulphate concentrations of the exposure solutions. At low sulphate concentrations (6 g/l), thaumasite formation is accelerated by the presence of 0.5% sodium chloride, but damage is reduced when the chloride concentration is increased to 2%[71]. However, at artificially high sulphate concentrations (20g/l), deterioration of limestone cements was reduced in the presence of 3.5% sodium chloride solution (21.14 g/l Cl)[67], but deterioration of limestone cements blended with aluminate-rich SCMs was slightly increased.
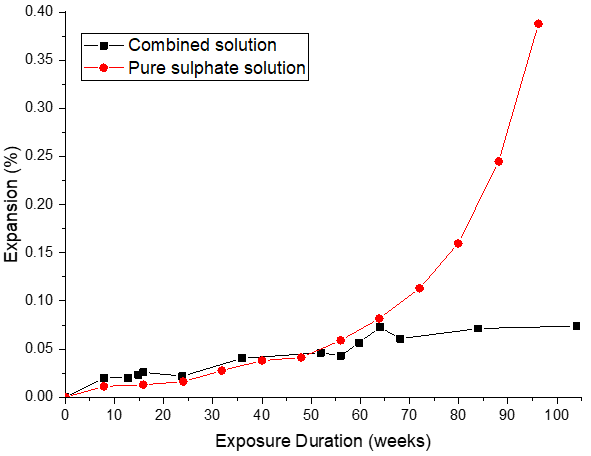
Figure 4. Comparison between expansion of CEM I mortar prisms exposed to pure sulphate[72] and combined chloride–sulphate solution[69].
3.2. Effect of Sulphate on Chloride Diffusion
In addition to the effect of chlorides on sulphate-induced expansion, sulphates can have a clear impact on chloride ingress. The formation of ettringite upon exposure densifies the hardened cement paste matrix, and has been suggested to reduce chloride ingress. However, at later ages, ettringite formation can lead to cracking of the concrete, facilitating chloride ingress[71][73]. Typical data are shown in Table 3[74][69][75] showing chloride ingress upon exposure to sodium chloride and combined sodium chloride–sulphate solutions.
However, results are not conclusive and some studies have shown increased total chloride penetration in seawater compared to pure sodium chloride solution[3], and possible acceleration of chloride ingress up to 30 weeks has been reported upon exposure to very high salt concentrations, but beyond 30 weeks and prior to any visible cracking, chloride ingress was reduced[76].
Table 3. Comparisons between chloride diffusion in mortars exposed by submersion to pure chloride and combined chloride–sulphate solutions from different authors[74][69][75].
|
Curing Duration (Days) |
20 °C |
20 °C Maes & De Belie [75] |
38 °C |
|||||
|
Binder/Temperature |
Da (m2/S) |
Da (m2/S) |
Binder/Temperature |
Da (m2/S) |
||||
|
Pure Cl |
Combined |
Pure Cl |
Combined |
Pure Cl |
Combined |
|||
|
(×10−12) |
(×10−12) |
(×10−12) |
(×10−12) |
(×10−12) |
(×10−12) |
|||
|
7 |
C1-20 °C |
51.50 |
1.40 |
- |
- |
C1-38 °C |
44.10 |
2.03 |
|
7 |
30S1-20 °C |
5.41 |
0.76 |
- |
- |
30S1-38 °C |
3.56 |
0.67 |
|
28 |
C1-20 °C |
17.50 |
1.43 |
5.27 |
3.72 |
C1-38 °C |
7.92 |
2.01 |
|
28 |
30S1-20 °C |
3.87 |
0.64 |
2.94 |
2.55 |
30S1-38 °C |
2.47 |
0.94 |
3.3. Effect of Sulphate on Chloride Binding Capacity
As mentioned previously, aluminates preferentially bind with sulphates over chlorides, forming ettringite at the expense of Friedel’s salt. This can decrease chloride binding. However, the extent of this appears dependent on the binder composition, with aluminate-rich SCMs providing some resilience and maintaining chloride binding capacity.
Combined sulphate–chloride exposure has been found to exacerbate reinforcement corrosion. Al-Amoudi and Maslehuddin[77] showed that steel corrosion was greater in combined chloride–sulphate solutions than in either pure chloride or sulphate solutions. Similarly, Dewah et al.[78] concluded that long-term corrosion current density in chloride solutions increased in the presence of sulphate.
3.4. Effect of Exposure Conditions
Exposure conditions have significant effects on the deteriorations caused by chloride and sulphate attack, whether in combination or in isolation. Cyclic wetting and drying conditions are more deleterious than permanent submersion due to the build-up of salts during drying stages[9][79][80][81]. This is well-noted and classified accordingly in the European Specification for concrete EN 206[12]. However, variations in compositions of the combined chloride–sulphate solutions, as highlighted in Table 4, make comparisons across different findings difficult.
Table 4. Selected recent studies to highlight differences in binder compositions and methodologies.
|
Binder Compositions |
Methods Employed to Study Chloride Attack |
Methods Employed to Study Sulphate Attack |
Compositions of Exposure Solutions |
References |
|
PC, LS, NP, FA, MK. GGBS |
|
Visual inspection, mass measurements, Compressive strengths, XRD |
21.14 g/L Cl + 20 g/L SO4 |
[67] |
|
PC, SF, FA, GGBS |
XRD, Titration |
|
5% Na2SO4, KSO4, MgSO4 |
[82] |
|
PC, APS, FA |
|
Flexural strength, SEM, XRD, MIP |
24,530 ppm-NaCl, 4090 ppm-Na2SO4 |
[65] |
|
PC |
XRF, degree of hydration, chloride binding isotherms, titration, SEM-EDX |
|
MgCl2, NaCl, NaCl + MgCl2, MgSO4 + MgCl2 |
[83] |
|
PC, GGBS, HSR |
Cl diffusion, Cl colour boundary, |
mass change, length change, XRD |
165 g/L NaCl, 27.5 g/L Na2SO4 |
[75] |
|
PC, LS filler |
|
Infra-red spectroscopy, XRD, SEM, Mass change |
5–20 g/L NaCl, 6 g/L MgSO47H2O |
[71] |
|
PC, 50% GGBS, 30% FA |
|
DME, mass change, XRD, TCC, TGA/DSC, MIP wetting/drying |
5% NaCl, 5, 10% Na2SO4 |
[84] |
|
PC, Portland pozzolana cement |
XRD, FTIR, EDX, potentiodynamic polarization |
|
3–7% NaCl, 3–12% MgSO4, Na2SO4 |
[85] |
|
PC, CAC, GGBS |
MIP, XRD, TCC |
mass-change, compressive strength, |
5% NaCl + 5% Na2SO4 |
[86] |
|
PC, FA |
Titration, TCC, coupled chloride-sulphate diffusion models |
Titration, coupled chloride-sulphate diffusion models |
10% NaCl + 5% Na2SO4 |
[87] |
|
PC |
|
Compressive strength, length change, mass change, SEM, EDX, TG(DTG/DSC) and XRD |
3–10% Na2SO4 + 3% NaCl |
[7] |
|
PC |
|
TCC, XRD, SEM, EDX, MIP |
25% NaCl + 5% Na2SO4 |
[76] |
|
PC |
Diffusion, Numerical Model |
Diffusion, Numerical Model |
0.5% Na2SO4 + 0.4–0.8% NaCl |
[88] |
Notes: PC—Portland cement; LS—Limestone; NP—Natural pozzolana; FA—Fly ash; MK—Metakaolin; GGBS—Ground granulated blast-furnace slag; SF—Silica fume; APS—Activated paper sludge; HSR—High-sulphate-resistant cement; CAC—Calcium aluminate cement; TCC—Total chloride content; DME—Dynamic modulus of elasticity; FTIR—Fourier-Transform Infrared Spectroscopy; MIP—Mercury Intrusion Porosimetry; XRD—X-ray diffraction; SEM-EDX—Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy; TGA—Thermogravimetric analysis.
4. Summary
- The rate of chloride ingress into concrete is dependent on several factors, such as the pore structure of the concrete matrix, curing conditions, w/b ratio, use of supplementary cementitious materials (SCMs) and the chloride binding ability of the cementitious materials in the concrete matrix.
- Chloride binding improves concrete durability by removing chloride ions from the pore solution that could otherwise initiate chloride-induced corrosion of steel reinforcement. Hence, higher chloride binding capacity reduces corrosion risk.
- Several factors, such as the cement type, alkalinity of the pore solution, cation type of the salt, chloride concentration, temperature, presence of other anions, and the use of SCMs influence chloride binding. The increased aluminate contents of SCMs improve chloride binding, as does higher C3A contents in cements.
- Environmental factors also affect chloride binding, it being reduced at higher pH, in the presence of sodium ions, or other anions. Meanwhile, chloride binding increases with the concentration of the chloride solution.
- Exposure to combined chloride–sulphate solutions can lead to reduced chloride binding, thus, ultimately leaving reinforcement more susceptible to corrosion. However, in the short term, sulphate ions can hinder chloride penetration due to ettringite formation. Conversely, the presence of sodium chloride tends to mitigate sodium sulphate attack.
This entry is adapted from the peer-reviewed paper 10.3390/app13010182
References
- Galan, I.; Glasser, F.P. Chloride in cement. Adv. Cem. Res. 2015, 27, 63–97, doi:doi:10.1680/adcr.13.00067.
- Ukpata, J.O.; Basheer, P.A.M.; Black, L. Performance of plain and slag-blended cements and mortars exposed to combined chloride–sulfate solution. Adv. Cem. Res. 2018, 30, 371–386, doi:10.1680/jadcr.17.00121.
- De Weerdt, K.; Lothenbach, B.; Geiker, M.R. Comparing chloride ingress from seawater and NaCl solution in Portland cement mortar. Cem. Concr. Res. 2019, 115, 80–89.
- Sun, H.; Liu, S.; Cao, K.; Yu, D.; Memon, S.A.; Liu, W.; Zhang, X.; Xing, F.; Zhao, D. Degradation mechanism of cement mortar exposed to combined sulfate–chloride attack under cyclic wetting–drying condition. Mater. Struct. 2021, 54, 1–17.
- Whittaker, M.; Black, L. Current knowledge of external sulfate attack. Adv. Cem. Res. 2015, 27, 532–545, doi:10.1680/adcr.14.00089.
- Ukpata, J.O.; Basheer, P.A.M.; Black, L. Slag hydration and chloride binding in slag cements exposed to a combined chloride-sulphate solution. Constr. Build. Mater. 2019, 195, 238–248.
- Zhao, G.; Li, J.; Shi, M.; Cui, J.; Xie, F. Degradation of cast-in-situ concrete subjected to sulphate-chloride combined attack. Constr. Build. Mater. 2020, 241, 117995.
- Ju, X.; Wu, L.; Lin, C.; Yang, X.; Yang, C. Prediction of chloride concentration with elevation in concrete exposed to cyclic drying-wetting conditions in marine environments. Constr. Build. Mater. 2021, 278, 122370.
- Ben Fraj, A.; Bonnet, S.; Khelidj, A. New approach for coupled chloride/moisture transport in non-saturated concrete with and without slag. Constr. Build. Mater. 2012, 35, 761–771.
- Ghazy, A.; Bassuoni, M.T. Response of concrete to cyclic environments and chloride-based salts. Mag. Concr. Res. 2019, 71, 533–547.
- Tang, X.; Xu, Q.; Qian, K.; Ruan, S.; Lian, S.; Zhan, S. Effects of cyclic seawater exposure on the mechanical performance and chloride penetration of calcium sulfoaluminate concrete. Constr. Build. Mater. 2021, 303, 124139.
- BSEN206 Concrete — Specification, performance, production and conformity; CEN: United Kingdom, 2013;
- Nagesh, M.; Bhattacharjee, B. Modelling of chloride diffusion in concrete and determination of diffusion coefficients. ACI Mater. J. 1998, 95, 113–120.
- Bastidas-Arteaga, E.; Chateauneuf, A.; Sánchez-Silva, M.; Bressolette, P.; Schoefs, F. A comprehensive probabilistic model of chloride ingress in unsaturated concrete. Eng. Struct. 2011, 33, 720–730.
- Nielsen, E.P.; Geiker, M.R. Chloride diffusion in partially saturated cementitious material. Cem. Concr. Res. 2003, 33, 133–138.
- Olsson, N.; Baroghel-Bouny, V.; Nilsson, L.-O.; Thiery, M. Non-saturated ion diffusion in concrete – A new approach to evaluate conductivity measurements. Cem. Concr. Compos. 2013, 40, 40–47, doi:10.1016/j.cemconcomp.2013.04.001.
- Yang, C.C.; Cho, S.W.; Wang, L.C. The relationship between pore structure and chloride diffusivity from ponding test in cement-based materials. Mater. Chem. Phys. 2006, 100, 203–210, doi:10.1016/j.matchemphys.2005.12.032.
- Chen, H.J.; Huang, S.S.; Tang, C.W.; Malek, M.A.; Ean, L.W. Effect of curing environments on strength, porosity and chloride ingress resistance of blast furnace slag cement concretes: A construction site study. Constr. Build. Mater. 2012, 35, 1063–1070.
- Chidiac, S.E.; Shafikhani, M. Phenomenological model for quantifying concrete chloride diffusion coefficient. Constr. Build. Mater. 2019, 224, 773–784, doi:https://doi.org/10.1016/j.conbuildmat.2019.07.006.
- Güneyisi, E.; Özturan, T.; Gesogˇlu, M. Effect of initial curing on chloride ingress and corrosion resistance characteristics of concretes made with plain and blended cements. Build. Environ. 2007, 42, 2676–2685, doi:10.1016/j.buildenv.2006.07.008.
- Ogirigbo, O.R.; Black, L. Chloride binding and diffusion in slag blends: Influence of slag composition and temperature. Constr. Build. Mater. 2017, 149, 816–825.
- Jiang, P.; Jiang, L.; Zha, J.; Song, Z. Influence of temperature history on chloride diffusion in high volume fly ash concrete. Constr. Build. Mater. 2017, 144, 677–685.
- Page, C.L.; Short, N.R.; El Tarras, A. Diffusion of chloride ions in hardened cement pastes. Cem. Concr. Res. 1981, 11, 395–406.
- Jaegermann, C. Effect of water-cement ratio and curing on chloride penetration into concrete exposed to Mediterraean Sea climate. ACI Mater. J. 1990, 87, 333–339.
- Arya, C.; Xu, Y. Effect of cement type on chloride binding and corrosion of steel in concrete. Cem. Concr. Res. 1995, 25, 893–902.
- Dhir, R.K.; El-Mohr, M.A.K.; Dyer, T.D. Developing chloride resisting concrete using PFA. Cem. Concr. Res. 1997, 27, 1633–1639.
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7, doi:10.1016/j.cemconres.2011.01.001.
- Wang, D.; Zhou, X.; Fu, B.; Zhang, L. Chloride ion penetration resistance of concrete containing fly ash and silica fume against combined freezing-thawing and chloride attack. Constr. Build. Mater. 2018, 169, 740–747, doi:https://doi.org/10.1016/j.conbuildmat.2018.03.038.
- Correia, V.; Gomes Ferreira, J.; Tang, L.; Lindvall, A. Effect of the addition of ggbs on the frost scaling and chloride migration resistance of concrete. Appl. Sci. 2020, 10, 3940.
- Goñi, S.; Frias, M.; Vigil de la Villa, R.; García, R. Sodium chloride effect on durability of ternary blended cement. Microstructural characterization and strength. Compos. Part B Eng. 2013, 54, 163–168.
- Birnin-Yauri, U.A.A.; Glasser, F.P.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723.
- Rasheeduzzafar; Hussain, S.E.; Al-Saadoun, S.S. Effect of tricalcium aluminate content of cement on chloride binding and corrosion of reinforcing steel in concrete. ACI Mater. J. 1992, 89, 3–12.
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. The binding of chloride ions by sulphate resistant portland cement. Cem. Concr. Res. 1995, 25, 581–592.
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment – A review. Constr. Build. Mater. 2009, 23, 1–13.
- Sandberg, P. Studies of chloride binding in concrete exposed in a marine environment. Cem. Concr. Res. 1999, 29, 473–477.
- Delagrave, A.; Marchand, J.; Ollivier, J.P.; Julien, S.; Hazrati, K. Chloride binding capacity of various hydrated cement systems. Adv. Cem. Based Mater. 1997, 6, 28–35.
- Arya, C.; Buenfeld, N.R.; Newman, J.B. Factors influencing chloride-binding in concrete. Cem. Concr. Res. 1990, 20, 291–300.
- Shi, Z.; Geiker, M.R.; De Weerdt, K.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement–metakaolin–limestone blends. Cem. Concr. Res. 2017, 95, 205–216.
- Dhir, R.K.; El-Mohr, M.A.K.; Dyer, T.D. Chloride binding in GGBS concrete. Cem. Concr. Res. 1996, 26, 1767–1773.
- Luo, R.; Cai, Y.; Wang, C.; Huang, X. Study of chloride binding and diffusion in GGBS concrete. Cem. Concr. Res. 2003, 33, 1–7.
- Niu, D.; Sun, C. Study on interaction of concrete carbonation and chloride corrosion. Kuei Suan Jen Hsueh Pao/Journal Chinese Ceram. Soc. 2013, 41, 1094–1099.
- Suryavanshi, A.K.; Swamy, N.R. Stability of Friedel’s salt in carbonated concrete structural elements. Cem. Concr. Res. 1996, 26, 729–741.
- Cheng, A.; Huang, R.; Wu, J.K.; Chen, C.H. Influence of GGBS on durability and corrosion behavior of reinforced concrete. Mater. Chem. Phys. 2005, 93, 404–411.
- Kayali, O.; Khan, M.S.H.; Sharfuddin Ahmed, M. The role of hydrotalcite in chloride binding and corrosion protection in concretes with ground granulated blast furnace slag. Cem. Concr. Compos. 2012, 34, 936–945.
- Khan, M.S.H.; Kayali, O.; Troitzsch, U. Chloride binding capacity of hydrotalcite and the competition with carbonates in ground granulated blast furnace slag concrete. Mater. Struct. 2016, 49, 4609–4619.
- Xu, Y. The influence of sulphates on chloride binding and pore solution chemistry. Cem. Concr. Res. 1997, 27, 1841–1850.
- Ogirigbo, O.R.; Black, L. Chloride binding of GGBS concrete: influence of aluminium content, added sulphate and temperature. In Proceedings of the Ibausil International conference on Building Materials; Weimar, 2015; pp. 1506–1513.
- Florea, M.V.A.; Brouwers, H.J.H. Modelling of chloride binding related to hydration products in slag-blended cements. Constr. Build. Mater. 2014, 64, 421–430.
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Long term performance of chloride binding capacity in fly ash concrete in a marine environment. Constr. Build. Mater. 2010, 24, 1352–1357, doi:10.1016/j.conbuildmat.2009.12.039.
- Liu, J.; Liu, J.; Huang, Z.; Zhu, J.; Liu, W.; Zhang, W. Effect of fly ash as cement replacement on chloride diffusion, chloride binding capacity, and micro-properties of concrete in a water soaking environment. Appl. Sci. 2020, 10, 6271.
- Qiao, C.; Suraneni, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23° C. Cem. Concr. Compos. 2019, 97, 43–53.
- Kayyali, O.A.; Qasrawi, M.S. Chloride binding capacity in cement-fly-ash pastes. J. Mater. Civ. Eng. 1992, 4, 16–26.
- Uysal, M.; Akyuncu, V. Durability performance of concrete incorporating Class F and Class C fly ashes. Constr. Build. Mater. 2012, 34, 170–178, doi:https://doi.org/10.1016/j.conbuildmat.2012.02.075.
- Page, C.L.; Vennesland, Ø. Pore solution composition and chloride binding capacity of silica-fume cement pastes. Matériaux Constr. 1983, 16, 19–25.
- Mangat, P.S.; Molloy, B.T. Chloride binding in concrete containing PFA, GBS or silica fume under sea-water exposure. Mag. Concr. Res. 1995, 47, 129–141.
- Byfors, K.; Hansson, C.M.; Tritthart, J. Pore solution expression as a method to determine the influence of mineral additives on chloride binding. Cem. Concr. Res. 1986, 16, 760–770.
- Talib, A.Y.; Rasheeduzzafar; Al-Gahtani, A.S. Effect of Temperature on the Chloride Binding Capacity of Silica Fume Blended Cernent. In Proceedings of the International Conference on Corrosion and Corrosion Protection of Steel in Concrete; Sheffield, 1994; pp. 806–816.
- Hornain, H.; Marchand, J.; Duhot, V.; Moranville-Regourd, M. Diffusion of chloride ions in limestone filler blended cement pastes and mortars. Cem. Concr. Res. 1995, 25, 1667–1678.
- UNEP; Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26.
- Adu-Amankwah, S.; Rahmon, S.; Black, L. From composition to the microstructure and durability of limestone ternary blended cements: A systematic review. Adv. Cem. Res. 2022, 34, 206–224.
- Ipavec, A.; Vuk, T.; Gabrovšek, R.; Kaučič, V. Chloride binding into hydrated blended cements: The influence of limestone and alkalinity. Cem. Concr. Res. 2013, 48, 74–85.
- Mohammed, T.U.; Hamada, H. Relationship between free chloride and total chloride contents in concrete. Cem. Concr. Res. 2003, 33, 1487–1490.
- Tang, L.; Nilsson, L.-O. Chloride binding capacity and binding isotherms of OPC pastes and mortars. Cem. Concr. Res. 1993, 23, 247–253.
- 63. Zibara, H. Binding of External Chlorides by Cement Pastes. Ph.D Thesis, University of Toronto, Toronto, ON, Canada, 2001
- Frias, M.; Goñi, S.; García, R.; Villa, R.V. d. L.V. de La Seawater effect on durability of ternary cements. Synergy of chloride and sulphate ions. Compos. Part B Eng. 2013, 46, 173–178, doi:10.1016/j.compositesb.2012.09.089.
- Holden, W.R.R.; Page, C.L.L.; Short, N.R.R. The influence of chlorides and sulphates on durabilty. In Corrosion of reinforcement in concrete; Crane, A.P., Ed.; Ellis Horwood Limited: London, 1983; pp. 143–150.
- Sotiriadis, K.; Nikolopoulou, E.; Tsivilis, S.; Pavlou, A.; Chaniotakis, E.; Swamy, R.N. The effect of chlorides on the thaumasite form of sulfate attack of limestone cement concrete containing mineral admixtures at low temperature. Constr. Build. Mater. 2013, 43, 156–164.
- Harrison, W.H. Effect of chloride in mix ingredients on sulphate resistance of concrete. Mag. Concr. Res. 1990, 42(152), 113–126.
- Ukpata, J.O. Durability of slag-blended cements in composite chloride-sulphate environments, University of Leeds, 2018.
- Al-Amoudi, O.S.B.; Rasheeduzzafar; Maslehuddin, M.; Abduljauwad, S.N. Influence of chloride ions on sulphate deterioration in plain and blended cements. Mag. Concr. Res. 1994, 46, 113–123.
- Abdalkader, A.H.M.; Lynsdale, C.J.; Cripps, J.C.J.C. The effect of chloride on cement mortar subjected to sulfate exposure at low temperature. Constr. Build. Mater. 2015, 78, 102–111, doi:http://dx.doi.org/10.1016/j.conbuildmat.2014.12.006.
- Whittaker, M.J. The Impact of Slag Composition on the Microstructure of Composite Slag Cements Exposed to Sulfate Attack, University of Leeds: Leeds, UK, 2014.
- Zuquan, J.; Wei, S.; Yunsheng, Z.; Jinyang, J.; Jianzhong, L. Interaction between sulfate and chloride solution attack of concretes with and without fly ash. Cem. Concr. Res. 2007, 37, 1223–1232.
- Ogirigbo, O.R. Influence of slag composition and temperature on the hydration and performance of slag blends in chloride environments, University of Leeds: Leeds, 2016, Vol. PhD.
- Maes, M.; De Belie, N. Resistance of concrete and mortar against combined attack of chloride and sodium sulphate. Cem. Concr. Compos. 2014, 53, 59–72, doi:http://dx.doi.org/10.1016/j.cemconcomp.2014.06.013.
- Cao, Y.; Guo, L.; Chen, B. Influence of sulfate on the chloride diffusion mechanism in mortar. Constr. Build. Mater. 2019, 197, 398–405.
- Al-Amoudi, O.S.B.; Maslehuddin, M. The effect of chloride and sulfate ions on reinforcement corrosion. Cem. Concr. Res. 1993, 23, 139–146.
- Dewah, H.A.F.; Maslehuddin, M.; Austin, S.A. Long-term effect of sulfate ions and associated cation type on chloride-induced reinforcement corrosion in Portland cement concretes. Cem. Concr. Compos. 2002, 24, 17–25.
- Hong, K.; Hooton, R.D. Effects of cyclic chloride exposure on penetration of concrete cover. Cem. Concr. Res. 1999, 29, 1379–1386.
- Thomas, M.D.A.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cem. Concr. Res. 1999, 29, 487–495.
- Shi, X.; Yang, Z.; Liu, Y.; Cross, D. Strength and corrosion properties of Portland cement mortar and concrete with mineral admixtures. Constr. Build. Mater. 2011, 25, 3245–3256.
- Xu, J.; Zhang, C.; Jiang, L.; Tang, L.; Gao, G.; Xu, Y. Releases of bound chlorides from chloride-admixed plain and blended cement pastes subjected to sulfate attacks. Constr. Build. Mater. 2013, 45, 53–59.
- De Weerdt, K.; Orsáková, D.; Geiker, M.R. The impact of sulphate and magnesium on chloride binding in Portland cement paste. Cem. Concr. Res. 2014, 65, 30–40.
- Chen, Y.; Gao, J.; Tang, L.; Li, X. Resistance of concrete against combined attack of chloride and sulfate under drying–wetting cycles. Constr. Build. Mater. 2016, 106, 650–658.
- Shaheen, F.; Pradhan, B. Influence of sulfate ion and associated cation type on steel reinforcement corrosion in concrete powder aqueous solution in the presence of chloride ions. Cem. Concr. Res. 2017, 91, 73–86, doi:https://doi.org/10.1016/j.cemconres.2016.10.008.
- Li, G.; Zhang, A.; Song, Z.; Liu, S.; Zhang, J. Ground granulated blast furnace slag effect on the durability of ternary cementitious system exposed to combined attack of chloride and sulfate. Constr. Build. Mater. 2018, 158, 640–648, doi:https://doi.org/10.1016/j.conbuildmat.2017.10.062.
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232.
- Sun, D.; Cao, Z.; Huang, C.; Wu, K.; De Schutter, G.; Zhang, L. Degradation of concrete in marine environment under coupled chloride and sulfate attack: a numerical and experimental study. Case Stud. Constr. Mater. 2022, e01218.
