Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Ketogenic diets (KD) encompass a lower consumption of carbohydrates, adequate protein, and a high fat regimen which induces ketone body production via mimicking the metabolism of the fasting state without significant calorie deprivation. Herein, several facets of ketogenic diet as an immunomodulator with respect to its expansive clinical applications are presented.
- ketogenic diet
- cancer
- immune modulation
- immunotherapy
- infection
1. Immune Modulation by Ketogenic Diet in Viral Infection
The COVID-19 pandemic has contributed to the deaths of more than 6 million people. Studies have provided the cellular mechanisms for understanding the composite SARS-CoV-2 access route on the cell’s surface in the host. SARS-CoV-2 binds to ACE2 receptors and excessively induces the secretion of TNF-α, IL-6, and IL-1 are pro-inflammatory cytokines, promoting the progression of acute respiratory distress syndrome (ARDS) [11]. A eucaloric ketogenic diets (KD) has been proposed to have potential therapeutic role against COVID-19 due to its role in suppressing critical risk complications, such as anti-inflammation, hypertension, type-2 diabetes, obesity, and metabolism modulation [12,13]. An MCTs-rich KD can provoke lipid metabolism switch, could disfavor infection and replication of virus, and can inhibit the cytokine storm [14]. This swapping of the host lipid metabolism can also be achieved by consumption of coconut-rich medium-chain fatty acids along with olive oil, followed by fasting for 8–12 h and a dinner rich with vegetables and fruits, resulting in activation of the ketogenic pathway [14].
Amongst the 68 COVID-19 patients who received a eucaloric standard diet, 34 patients receiving KD were observed to have a lower risk of mortality [15]. Ketone bodies like β-hydroxybutyrate (BHB) maintain the redox balance by providing an alternative carbon source for oxidative phosphorylation (OXPHOS) and the synthesis of bioenergetic amino acids and glutathione. KD induces levels of interferon-γ by CD4+. Under a SARS-CoV-2 stimulated ARDS stress, the exhausted and glycolysis skewed T cells can be reprogrammed metabolically by BHB to perform OXPHOS [16]. Activation of ketogenesis reduces pathogenic monocytes in the lungs of aged mice infected with mCoV-A59 (murine beta coronavirus infected with mouse hepatitis virus strain-A59), inactivates the NLRP3 inflammasome, and increases the tissue-protective γδ T cells [3,17,18]. There are many proposed molecular mechanisms that explain the therapeutic role of the exogenous ketone-based metabolic therapy in combination with a moderately high-fat diet against the cytokine storm induced by severe SARS-CoV-2 infection. By reducing glucose uptake into ILC2s, KD reduces lung inflammation. A study in mice proved that KD potentially activated a γδ T cell response, leading to decrease IAV mortality. KD is also known to normalize the disease induced upregulation of Th17/Treg Ratio [19]. In COVID-19 patients, KD has been proven to provide superior energy by directing human CD8+ T cells towards aerobic mitochondrial metabolism [20].
2. Ketogenic Diet Mediated Immune Regulation in Cancer
The ketogenic diet (KD) stimulates a metabolic switch from glycolysis into mitochondrial metabolism, the differential stress resistance phenomenon with high tumor control ability and lower normal-tissue complications, making it an intriguing dietary approach for cancer patients who are under the supervision and follow-up of a healthcare provider (Figure 1) [21]. Initial findings regarding the role of food consumption curtailment in tumor growth was reported by Rous in 1914 [22]. Later, Tisdale et al. (1987), [23] reported the anti-tumor effect of ketogenic diet. Afterwards, many researchers supported the utilization of the ketogenic diet in various animal models through numerous mechanisms [24,25,26].
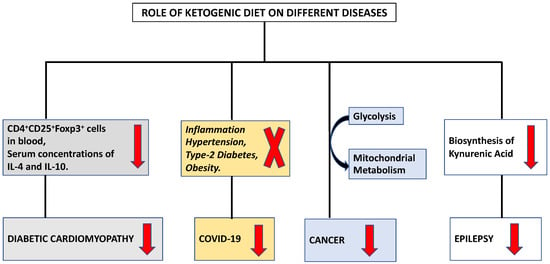
Figure 1. Modulation of different diseases by ketogenic diet.
The ketogenic diet was utilized for the management of human malignant brain tumor [27]. Researchers utilized an immunocompetent mouse model of malignant glioma and observed that a ketogenic diet led to blood glucose reduction, blood ketones elevation, and overall life extension [28,29]. CTLA-4 (cytotoxic T lymphocytes associated antigen 4) and PD-1 (Programmed cell death protein-1) are the immune checkpoints utilized as targets for treatment of multiple tumor types [30]. By lowering PD-L1 protein levels and enhancing the expression of type I interferon and antigen presentation genes, the introduction of the ketogenic diet increases the effectiveness of anti-CTLA-4 immunotherapy [31] resulting in anti-proliferative effects due to cell cycle extension.
IFN induced chemokines, such as CXCL-9, CXCL-10, and CXCL-11, can upregulate T-cell infiltration in melanoma [32] and CT26+ mouse model [33], thus enhancing immunity against tumor. Moreover, 3-hydroxybutyrate, a principal ketone body generated via ketogenic diet consumption was reported to induce T-cell dependent tumor growth interruption in belligerent tumor models [34]. In a glioblastoma mouse model, the ketogenic diet enhanced anti-tumor primary and acquired immune response by promoting cytolysis mediated by CD8+ T cells and increased CD4+ T-cells infiltration together with T cell killing activity [35]. Further, Sun et al. 2022 [36] also demonstrated the inhibitory role of ketogenic diet on tumor growth by enhancing the Th1 cells and cellular immune function. An increase in CD8+ T-cells and decrease in CD4+ FOX P3+ T-cells in tumor tissue, attenuation of PD-L1, and CTL4 immunosuppression were the other observed mechanisms of action of the ketogenic diet in a tumor model [33].
Although PI3K enhanced the downstream of both insulin receptor and IGF-1R, PI3K/Akt dysregulation is directly linked to neoplasmic development and increased resistance to cancer therapy [37]. There are many different factors and micro-environments where mTOR signaling is modulated [38]. Growth factors, mitogens, PI3K, activated AMP kinase, and hormones such as insulin all operate as stimulants for mTOR signaling [38]. AMP-activated protein kinase (AMPK), phosphatidylinositide 3-kinase (PI3K), and mTOR are all adversely impacted by decreased nutritional patterns [39]. The tumor suppressor activity is increased by a ketogenic diet, which inhibits mTOR signaling by activating the AMPK signaling pathway [39]. Calorie restriction via the ketogenic diet decreased the expression of pro-inflammatory markers, including cyclooxygenase 2, nuclear factor-k, and macrophage inflammatory protein 2, in a mouse model of astrocytoma [40].
Macrophages are known to have regulatory functions in modulating tumor immune response. Studies reported that ketogenic diet utilization led to switch of tumor associated macrophage from M2 to M1 phenotype which inhibited tumor progression [41]. M1 macrophage can promote and amplify the Th1 type response inhibiting tumorigenesis [42]. Equilibrium between Th1/Th2 responses is crucial for cancer development as Th2 cytokines (IL-4, IL-5, IL-13) promote tumor growth and progression. An enhanced Th1/Th2 ratio was demonstrated by implementation of the ketogenic diet in a colon tumor allograft mouse model by up regulating the Th1 driven immune response and inflammatory response provided beneficial effects against tumor [33]. Although the ketogenic diet has several positive effects, it may not be able to stop tumors from developing, but it can postpone their growth and increase survival rates [43]. Furthermore, when used in conjunction with conventional radiotherapy or chemotherapy, KD exhibits a synergistic effect on the treatment of cancer [29,44].
3. Ketogenic Diet Mediated Immune Regulation in Cardiovascular Diseases
The four categories of cardiovascular disease, frequently referred as heart events, are as follows: Aortic atherosclerosis, cerebrovascular disease, peripheral artery disease, and coronary artery disease (CAD) [45,46,47]. Immune cells also play important role in heart failure, particularly to pathological CD4+ T-lymphocytes during ischemic heart failure and heart remodeling [48]. Humans practice fasting for various reasons including religious, ethical, health reasons etc. since ancient times. Visioli et al. (2022) concluded that dietary intake manipulation to reduce calorie intake, intermittent fasting, and prolonged fasting are included in human culture possibly because of their positive effect on health [49]. Evidence showing the relationship between the differentiation and functioning of immune cells with reference to nutrient metabolism is accumulating in the scientific literature. To induce ketosis without limiting fat intake, the keto diet, also known as the ketogenic diet, consists of a low-carbohydrate diet with a moderate amount of protein restriction [50].
A low carbohydrate diet for longer period depletes the glycogen store of the body and stimulate keto-genesis, making ketone bodies the only source of energy. In addition to serving as energy sources, these ketone bodies function as significant signaling molecules that influence the expression and activity of transcription factors including PGC-1 and sirtuins (SIRTs) [51,52], poly-adenosine diphosphate [ADP]-ribose polymerase 1 (PARP1), and ADP ribosyl cyclase [53] fibroblast growth factor 21 and nicotinamide adenine dinucleotide (NAD+) [54]. Additionally, calorie restriction inhibits the PI3K/Akt/mTOR axis while simultaneously activating the adenosine monophosphate-activated protein kinase (AMPK) and sirtuin family proteins [51,55,56].
Acetyl-coenzyme A carboxylase 1 (ACC1) activity is inhibited by AMPK, which prevents the production of fatty acids. ACC1 induces vascular endothelial cell impairment leading to increase in disease severity in acute ischemic stroke patients [57,58]. It also increases plasma triglyceride levels, which will lead to an enlargement of atherosclerotic plaque and vascular occlusion, and finally increases disease severity [59]. This alteration in lipid metabolism is linked to an increase in T regulatory cells and a decrease in Th17 cells, which together ameliorate brain ischemia [60]. Th17 cells influence blood pressure by producing IL-17 and IL-22. IL-17 may have an impact on the sodium transport system, which includes sodium chloride cotransporter, epithelial sodium channels, and the sodium-hydrogen exchanger, in the renal proximal and distal tubular epithelial cells 3 [61]. On the other hand, IL-22 may affect the cyclooxygenase of the cells of the vascular wall and increase endothelial dysfunction, in turn increasing resistance in blood flow [62]. Additionally, mice lacking Ƴδ T cells were reported to be protected from endothelial damage and hypertension caused by angiotensin II [63]. Fat-related illness symptoms have been linked to decreased pro-inflammatory Th17 cell numbers in the gut and adipose tissue [64].
The individual role of B cells was poorly understood in hypertension. It might occur due to the activation of B cells that need co-stimulatory signals from T cells. However, anti CD 20 therapy [65] and Taylor et al. (2018) [66] have shown an association in preventing angiotensin II- related hypertension in mice. IgG was accumulated in the aortic adventitia during Angiotensin II- dependent hypertension in mice. It is regarded as a significant site of collagen and macrophage accumulation [67]. Endothelial cell nitric oxide synthase relaxes vessels during hypertension. Mice fed on high fat diet expresses Fcγ receptors on endothelial cells. IgG after associating with antigen can target these Fcγ receptors of endothelial cells thus have a negative effect of vasorelaxing activity [68]. These findings indicate a role for B lymphocytes in the endothelial dysfunction that primes vascular stiffening and elevated blood pressure as well as vascular remodeling.
It has been established that a ketogenic diet is linked to enhanced cardiac function, cardiomyocyte survival, and decreased cardiac fibrosis [69,70]. According to investigation, the activation of cardiac fibroblasts by ketone bodies was increased by the activation of transforming growth factor-β1 [71]. The differentiation of group 2 innate lymphoid cells and the T cell subset is regulated by the ketogenic diet [72]. Additionally, ketogenesis functions as a novel metabolic pathway in CD8+ Tmem cells, modifying these cells to facilitate the creation of memories through improved mitochondrial performance and substrate metabolism [73]. IL 33 has been found to reduce the symptoms of cardiac fibrosis [74]. Tao et al. (2021) examined the function underlying mechanism of Ketogenic diet in diabetic cardiomyopathy [75]. They found a decrease in the level of both CD4+ CD25+ Foxp3+ cells in blood and serum concentrations of IL-4 and IL-10 (Figure 1). Ketone bodies prevented naive CD4+ T cells from differentiating into Tregs. In the presence of ketone bodies, ST2L ligand synthesis, the proportion of ST2L+ cells in Tregs, and IL-33 production all decreased. The NLRP3 inflammasome regulates the release of the pro-inflammatory cytokines IL-1 and IL-18 and caspase-1 activation in macrophages [76]. It is a crucial innate immune sensor that may become active in response to atherosclerosis [77]. Therefore, understanding the endogenous mechanisms that regulate the NLRP3 inflammasome’s deactivation may help in the management of a number of chronic disorders. The ketogenic diet reduces inflammation, and these anti-inflammatory effects may be associated with BHB facilitated inhibition of the NLRP3 inflammasome [78]. The immunomodulatory effects of KD on various immune cells are summarized in Table 1.
Table 1. The immunomodulatory effects of ketogenic diet on Immune Cells.
| Type of Ketogenic Diet (KD) | Mechanism of Action | Conclusions/Effects | References |
|---|---|---|---|
| Medium-Chain Triglycerides (MCT) | Reduced Leukocyte Count | Anti-Tumor Effect | [79] |
| Eucaloric Ketogenic Diet (EKD) | M1 Recruitment of Neutrophil and Platelets in Thrombo-Inflammation | Prevention of Cytokine Storm in COVID-19. | [80] |
| Very Low Calorie Ketogenic Diet (VLCKD) | Altered Leukocyte Methylation | Obesity Prevention | [81] |
| KD | Reduced WBC & Neutrophil Count | In Epilepsy, baseline immunosuppression does not worsen with KD. | [82] |
| KD | Enhanced activity of Th1 cells | Promoted cellular immune function in a CT26 colon tumor allografts mouse model. | [33] |
| KD | Suppression of Th1 & Th17 | Protection against autoinflammation (Central Nervous System). | [83] |
| KD | Suppression of macrophages & neutrophils | Attenuation of autoinflammation (Muckle-Wells syndrome/ Gout) | [83] |
| KD | Activation of protective γδ T cells and decreases myeloid cell subset | Ketogenesis-induced protection from mCoV-A59-driven inflammatory damage in aging. | [17] |
This entry is adapted from the peer-reviewed paper 10.3390/immuno3010001
This entry is offline, you can click here to edit this entry!
