Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The pomegranate fruit is made of white to deep purple seeds that are enclosed in a white, spongy, astringent membrane, also known as pericarp, covered by a thick red skin and a crown-shaped calyx. It contains a variety of beneficial ingredients, including flavonoids, ellagitannin, punicalagin, ellagic acid, vitamins, and minerals. Pomegranates possess numerous health benefits, and their use in disease treatment has been widely recognized since antiquity.
- pomegranate
- antioxidant
- respiratory
- diseases
1. Introduction
Phytotherapy is defined as the usage of plant-derived products in the treatment and prevention of diseases. Since the beginning of human civilization, medicinal plants have been used as pharmaceutics for the prevention and treatment of various diseases [1]. Medicinal plants play a major role in pharmaceutical industries as they contribute to the manufacturing of drugs [2]. Development of pharmaceutical drugs from plants reduces the use of synthetic antibiotics and increases life expectancy [3][4]. In the meantime, scientific interest in medicinal plants is increasing due to the high cost and side effects caused by allopathic drugs, in addition to the emergence of resistant microbial strains [5].
Pomegranate (Punica granatum L.) is derived from a deciduous tree in the Lythraceae family. According to reports, it first appeared in modern times in Iran and has since spread throughout the world. Since ancient times, it has been cultivated throughout the Mediterranean region and Northern India [6]. The pomegranate fruit is made of white to deep purple seeds that are enclosed in a white, spongy, astringent membrane, also known as pericarp, covered by a thick red skin and a crown-shaped calyx (Figure 1). This fruit is known to exhibit several biological properties, including antibacterial, anti-inflammatory, antioxidant, and anticancer activities [7][8]. The scientific studies on the health advantages of pomegranates that have been published over the past few decades demonstrate the scientific community’s intense interest in the fruit’s medicinal potential. Pomegranate has been used in a variety of medical systems for the treatment and therapy of a wide range of diseases and illnesses. For instance, it was recommended as an antiparasitic agent and as a treatment for diarrhea and ulcers in ancient Indian medicinal systems [9][10]. Its importance in the treatment of diabetes has been recognized in another traditional system, the Unani system of medicine [11].

Figure 1. Pomegranate fruit parts.
In addition, pomegranate and its constituents have been shown in studies to effectively affect a number of signaling pathways involved in inflammation, cellular transformation, hyperproliferation, angiogenesis, and the start of tumorigenesis, as well as suppressing the later stages of tumorigenesis and metastasis [12][13]. Pomegranate peel has been shown to inhibit a wide range of pathogens, including viruses, bacteria, fungi, and mold [14]. It has a great therapeutic effect on chronic inflammation, particularly digestive tract inflammation like ulcerative colitis [15]. Furthermore, all waste parts of the pomegranate fruit, such as the peel and seeds, can be processed into value-added products with industrial, medicinal, and cosmetic value [16].
Chronic respiratory diseases are one of the leading causes of death globally. Victims of lung disorders frequently experience long-term difficulties, with one of the major causes being treatment side effects as well as psychosocial struggles [17]. Respiratory diseases are characterized by unrestricted cell proliferation and no single defined cause, but they are associated with several risk factors, including tobacco use, infection, radiation exposure, air pollution, obesity, and alcohol consumption. Several epigenetic/environmental agents have been identified as key players in the development and progression of such diseases [18][19]. Despite significant advances in treatment options, the number of cases and deaths continues to rise, and millions of people die from these diseases globally [20][21].
2. Pomegranate in the Treatment of Respiratory Diseases
2.1. Pomegranate and Asthma
Asthma is a chronic condition in which the airways, due to inflammation, become narrow and swollen and are blocked by excess mucus. Treatment of asthma by inhalers can help control and minimize the symptoms, but their adverse effects ultimately limit their long-term use [22][23]. Studies have shown that pomegranate can play an effective medicinal role in asthma treatment. Eosinophils are involved in the development of asthma exacerbation and that IL-5 plays an important role in the activation and maturation of eosinophils [24]. Oliveira et al. revealed that the micro-encapsulated leaf extract from Punica granatum inhibited eosinophil recruitment to bronchoalveolar fluid and reduced the production of inflammatory cytokines such as IL-1b and IL-5 in the lungs of BALB/c mice used as asthma models [25]. Another study revealed that tannins extracted from the flower buds of P. granatum display an anti-histaminic activity that could contribute to its role as a traditional treatment for asthma [26]. This antihistaminic activity may open the door for future research studies that could evaluate the cumulative effect between pomegranate extracts and other antihistaminic drugs. All these findings depicted the effective role of pomegranate as a therapy for asthma; more studies are needed for exploring the exact mechanism behind this therapeutic effect.
2.2. Pomegranate and COPD
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder that causes airflow blockage and breathing-related problems. COPD is the third leading cause of mortality worldwide [27][28]. Cigarette smoking, indoor air pollution, and occupational dusts, fumes, and chemicals are important risk factors of COPD [29]. The exposure to cigarette smoke leads to severe oxidative damage to the lungs and to neutrophils recruitment via IL-1β and TNF-α. Neutrophils secrete proteases such as neutrophil elastase, caspases, and matrix metalloproteinases (MMPs), which break down the connective tissue in the lungs, resulting in emphysema [30]. Pomegranate has proven to be effective in treating COPD, even though the study done by Cerda et al. showed that pomegranate juice supplementation had no benefit in treating patients with stable COPD due to the metabolism of its polyphenols by the colonic microflora [31]. In fact, variations in polyphenol bioavailability and absorption could be possibly due to differences between individual and between species in gut microflora as well as differences in polyphenol structure [32][33]. Furthermore, in vitro research suggests that certain polyphenols in the colon may induce the production of conjugation enzymes, whereas in vivo research suggests that the composition of microbiota influences the capacity for producing enzymes required for conjugation [34][35]. External factors such as the food matrix in which polyphenols are consumed and health status can also influence polyphenol absorption efficiency. Age-related changes and metabolic disorders in the host have been shown to influence the distribution of intestinal microbiota, as well as the host’s ability to metabolize specific polyphenols and the types of conjugated forms produced [36][37][38]
Other research studies had proven the effectiveness of pomegranate in treating COPD. Husari et al., 2016, proved that pomegranate juice supplementation in animal models exposed to cigarette smoke reduced the emphysematous changes and attenuated the expression of inflammatory mediators such as IL-1β, IL-6, and TNF-α [39]. Accordingly, pomegranate reduces apoptosis and oxidative stress induced by cigarette smoke exposure in the lungs. In vitro, pomegranate juice inhibited the devastating effects of cigarette smoke on cultured human alveolar cells [39]. In conclusion, these studies proved that pomegranate can act as a potential treatment for COPD as it attenuates the damaging effects of cigarette smoke on the lungs.
2.3. Pomegranate and Influenza
Influenza is a viral disease that affects the upper and lower respiratory tract. It is caused by a wide range of influenza viruses. Some of these viruses infect humans, while others are specific to other species [40][41]. Some medicinal plants have been identified for use in treating influenza due to the failure of some synthetic drugs because of side effects [42][43]. Pomegranate has been shown to possess a therapeutic effect against influenza virus infections. Pomegranate polyphenol juice extract showed anti-influenza properties, as it had inhibited the replication of influenza A virus in Madin-Darby canine kidney cells (MDCK). Punicalagin was found to have virucidal effects, being the most effective anti-viral component of polyphenol extract. Punicalagin suppressed viral RNA replication and blocked the agglutination of chicken RBCs by the virus. Moreover, combining oseltamivir, an anti-influenza drug, with pomegranate polyphenol extract showed a synergistic effect [44]. Another study revealed that pomegranate polyphenols inhibited influenza virus infectivity. Electron microscopic analysis revealed that viral inactivation by pomegranate polyphenols was due to virion structural damage, with small changes in envelope glycoproteins [45]. Moradi et al. showed that the ethyl alcohol extract of pomegranate peel can suppress the replication of influenza A virus through inhibiting viral adsorption and internalization and viral RNA transcription [46][47]. Hence, pomegranate extracts must be further analyzed for therapeutic and prophylactic potential against influenza epidemics and pandemics.
2.4. Pomegranate and COVID-19
The extremely contagious respiratory illness COVID-19 is brought on by a new strain of coronavirus known as SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2) [48]. Coronavirus causes infections in the respiratory tract ranging from mild colds to severe acute respiratory distress syndrome [49]. The interaction of SARS-CoV-2 spike (S) transmembrane glycoprotein with the angiotensin-converting enzyme 2 (ACE-2) receptor on host cells is an essential step for virus entry and onset of infection [50]. Attenuating the binding ability of S-glycoprotein to ACE-2 receptor was one of the primary targets for treating COVID-19 disease [51]. Pomegranate was shown to be potential candidate for possible therapeutic application against COVID-19. A recent study showed that pomegranate peel extract significantly blocked the binding of S-glycoprotein to ACE-2 receptor [52]. Another computational study showed that punicalagin and punicalin from pomegranate peel extract inhibited the SARS-CoV-2 internalization process [53]. Punicalagin alone or its combination with Zn particles inhibited the activity of SARS-CoV-2 3CL protease in vitro [54]. 3CL protease is indispensable for disease progression and viral replication, as it cleaves the viral polyprotein to give a single useful protein [55][56]. Other studies had also shown that some anthocyanins and hydrolysable tannins may inhibit viral replication via binding with catalytic dyad residues of 3CL protease [57][58][59]. Medicinal plants rich in anthocyanins and tannins, such as pomegranate, can be used as natural anti-COVID-19 therapeutic agents (Figure 2). Moreover, a clinical case study revealed that the consumption of fresh pomegranate juice showed prophylactic and therapeutic effects against COVID-19 [60]. These findings support the efficacy of P. granatum L. as a therapeutic drug in the treatment of COVID-19 (Figure 2).
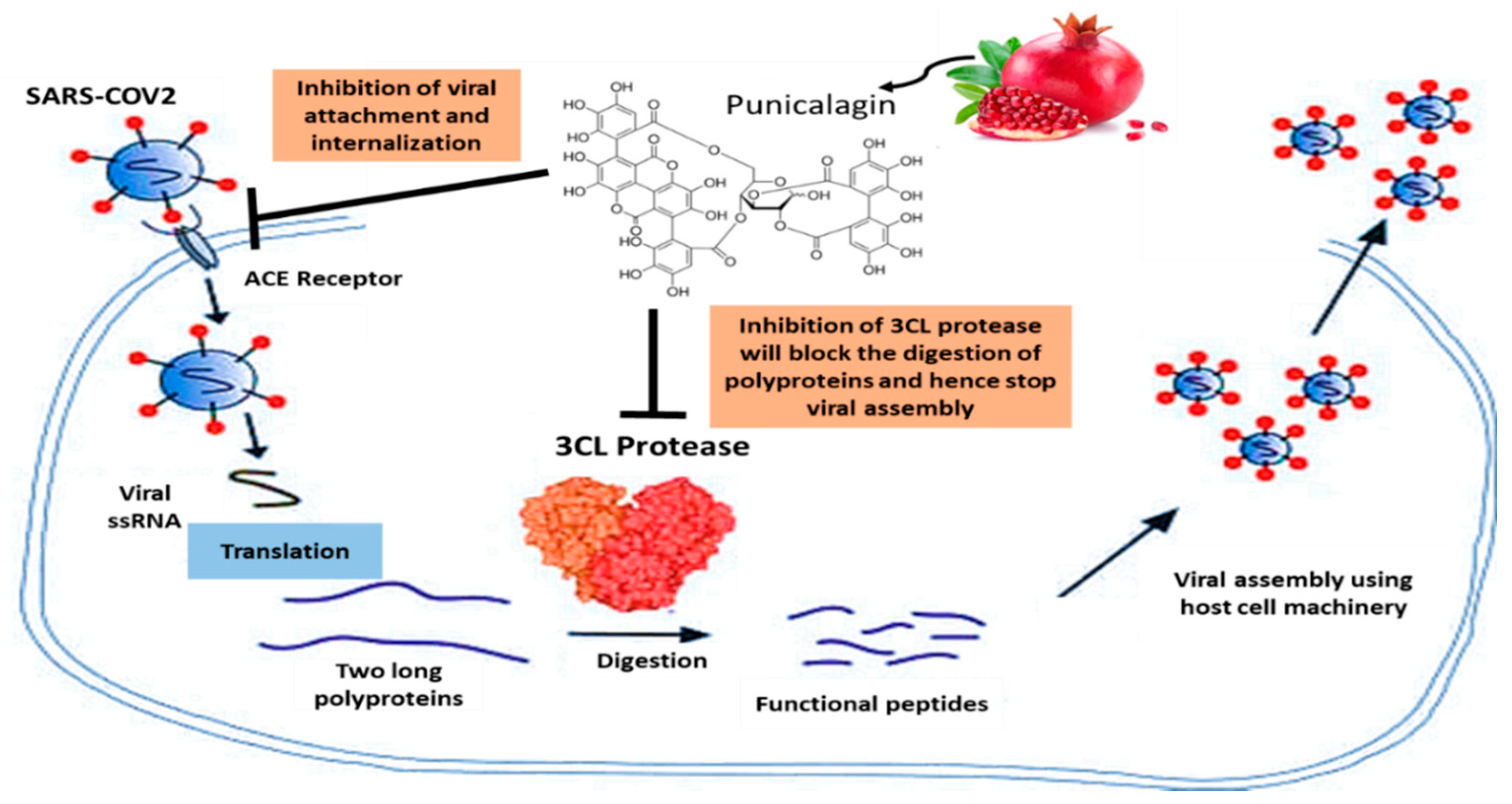
Figure 2. Pomegranate’s role in COVID-19.
2.5. Pomegranate and Lung Cancer
Lung cancer remains the leading cause of cancer-related mortality worldwide. The year 2020 recorded 2.2 million new lung cancer cases, accounting for 11.4% of the global cancer burden [61][62]. Many technical and pharmacological advances have been made in the staging and treatment of lung cancer, with the approval of newly synthesized chemotherapeutic drugs that improve the prognosis of patients diagnosed with metastatic tumors [63]. Yet these drugs are linked to many undesired side effects, which can be avoided by their substitution with natural products that have historically been invaluable as an origin of therapeutic agents [64][65]. Pomegranate has been shown to exhibit anti-cancerous effects against lung cancer in cell culture and in vivo studies. Punicalagin, an ellagitannin found in pomegranate peel, acts as an anti-proliferative agent on the A549 human lung carcinoma cell line. Punicalagin treatment induced apoptosis in A549 cells through mitochondria-mediated pathways without having any effect on normal lung fibroblast cells (MRC-5 cell line) [66]. Aqil et al. showed that punicalagin displays a strong anti-oxidant potential protecting against oxidative DNA damage and exhibits a strong anti-proliferative activity against lung cancer cells [67]. Punicalagin and its hydrolytic product (ellagic acid) showed cytotoxic effects on both human lung cancer cell lines A549 and H1299 [68]. Punicalagin inhibited STAT-3 translocation and accordingly induced apoptosis of A549 cells by inhibiting the expression of anti-apoptotic proteins (Bcl-2) and increasing the expression of the pro-apoptotic proteins (Bax, cytochrome C, caspase 9, and caspase 3) [69]. Another study investigated the anti-tumoral properties of pomegranate peel extract on A549 lung cancer cells. The authors showed that the peel extract reduced the cell viability, with a maximum growth inhibition of 80% at 250 µg/mL dose [70]. Moreover, the treatment of A549 cells with pomegranate fruit extract leads to a decrease in the viability of A549 cells with only minimal effects on normal human bronchial epithelial NHBE cells. Pomegranate fruit extract treatment leads to the G1 phase cell cycle arrest through the induction of WAF1/P21 and KIP1/P27, with consequent inhibition of cyclins D1, D2, and E as well as cyclin-dependent kinases cdk2, cdk4, and cdk6 [71]. In addition, treating A549 cells with pomegranate fruit extract resulted in the inhibition of MAPK and PI3K pathways as well as the NF-Kb pathway [71]. Yali Li et al. showed that pomegranate leaf extract exhibits anticancer effects through inhibiting the proliferation of A549 and H1299 non-small-cell lung carcinoma cell lines and mouse Lewis lung carcinoma cell line LL/2. Pomegranate leaf extract blocked the migration and invasion of H1299 cells via reducing the expression of metalloproteinases (MMPs), suggesting the effectiveness of pomegranate leaf extract in impairing metastasis [72]. Urothilin A, a major metabolite from pomegranate ellagitannins, was found to inhibit epithelial-to-mesenchymal transition (EMT) in lung cancer cells via decreasing the expression and activity of snail protein, an inducer of EMT [73]. Husari et al. showed that pomegranate juice supplementation to an animal model reduced the expression of HIF-1α and prevented the formation of lung nodules secondary to chronic cigarette smoke exposure, therefore decreasing the incidence of lung cancer [74]. These studies highlight the potential role of pomegranate fruit in the treatment of lung cancer (Figure 3).

Figure 3. Pomegranate activity in lung cancer.
This entry is adapted from the peer-reviewed paper 10.3390/app122312326
References
- Perez Gutierrez, R.; Baez, E. Cardioactive Agents from Plants. MRMC 2009, 9, 878–899.
- Rastogi, S.; Pandey, M.M.; Rawat, A.K.S. Traditional herbs: A remedy for cardiovascular disorders. Phytomedicine 2016, 23, 1082–1089.
- Li, Q.; Tu, Y.; Zhu, C.; Luo, W.; Huang, W.; Liu, W.; Li, Y. Cholinesterase, β-amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 2017, 108, 512–519.
- Nollet, L.M.L. (Ed.) Phenolic Compounds in Food: Characterization & Analysis; CRC Press is an imprint of the Taylor & Francis Group, an informa business; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-2296-4.
- Konaté, K.; Hilou, A.; Mavoungou, J.; Lepengué, A.; Souza, A.; Barro, N.; Datté, J.Y.; M’Batchi, B.; Nacoulma, O. Antimicrobial activity of polyphenol-rich fractions from Sida alba L. (Malvaceae) against co-trimoxazol-resistant bacteria strains. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 5.
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. 2008, 13, 128–144.
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057.
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405.
- Naqvi, S.; Khan, M.; Vohora, S. Antibacterial, antifungal and anthelmintic studies on Ochrocarpus longifolius. Planta Med. 1976, 29, 98–100.
- Cáceres, A.; Girón, L.M.; Alvarado, S.R.; Torres, M.F. Screening of antimicrobial activity of plants popularly used in guatemala for the treatment of dermatomucosal diseases. J. Ethnopharmacol. 1987, 20, 223–237.
- Saxena, A.; Vikram, N.K. Role of Selected Indian Plants in Management of Type 2 Diabetes: A Review. J. Altern. Complement. Med. 2004, 10, 369–378.
- Faria, A.; Calhau, C. The Bioactivity of Pomegranate: Impact on Health and Disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634.
- Khan, N.; Afaq, F.; Mukhtar, H. Cancer Chemoprevention Through Dietary Antioxidants: Progress and Promise. Antioxid. Redox Signal. 2008, 10, 475–510.
- Chen, J.; Liao, C.; Ouyang, X.; Kahramanoğlu, I.; Gan, Y.; Li, M. Antimicrobial Activity of Pomegranate Peel and Its Applications on Food Preservation. J. Food Qual. 2020, 2020, 8850339.
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. CPD 2019, 25, 1817–1827.
- Dhumal, S.S.; Karale, A.R.; Jadhav, S.B.; Kad, V.P. Recent Advances and the Developments in the Pomegranate Processing and Utilization: A Review. J. Agric. Crop Sci. 2014, 1, 1–7.
- Tereso, A.; Carreto, L.; Baptista, M.; Almeida, M.A. Interstitial Lung Disease Induced by Crizotinib in Non-Small-Cell Lung Cancer. Acta Med. Port. 2019, 32, 236–239.
- Bartling, B.; Hofmann, H.-S. Reduced proliferation capacity of lung cells in chronic obstructive pulmonary disease. Z. Gerontol. Geriat. 2019, 52, 249–255.
- Kolb, M.; Vašáková, M. The natural history of progressive fibrosing interstitial lung diseases. Respir. Res. 2019, 20, 57.
- Abegunde, D.O.; Mathers, C.D.; Adam, T.; Ortegon, M.; Strong, K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007, 370, 1929–1938.
- Obi, J.; Mehari, A.; Gillum, R. Mortality Related to Chronic Obstructive Pulmonary Disease and Co-morbidities in the United States, A Multiple Causes of Death Analysis. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 200–205.
- Scichilone, N. Asthma Control: The Right Inhaler for the Right Patient. Adv. Ther. 2015, 32, 285–292.
- Heffler, E.; Madeira, L.N.G.; Ferrando, M.; Puggioni, F.; Racca, F.; Malvezzi, L.; Passalacqua, G.; Canonica, G.W. Inhaled Corticosteroids Safety and Adverse Effects in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 776–781.
- Nakagome, K.; Nagata, M. Involvement and Possible Role of Eosinophils in Asthma Exacerbation. Front. Immunol. 2018, 9, 2220.
- de Oliveira, J.F.F.; Garreto, D.V.; da Silva, M.C.P.; Fortes, T.S.; de Oliveira, R.B.; Nascimento, F.R.F.; Da Costa, F.B.; Grisotto, M.A.G.; Nicolete, R. Therapeutic potential of biodegradable microparticles containing Punica granatum L. (pomegranate) in murine model of asthma. Inflamm. Res. 2013, 62, 971–980.
- Sunil, A.; Dhasade, V.; Patil, M.; Pal, S.; Subhash, C.; Barwal, S. Antihistaminic effect of various extracts of Punica granatum Linn. flower buds. J. Young Pharm. 2009, 1, 322.
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. 2018, 3, e4.
- Raherison, C.; Girodet, P.-O. Epidemiology of COPD. Eur. Respir. Rev. 2009, 18, 213–221.
- Berry, C.E.; Wise, R.A. Mortality in COPD: Causes, Risk Factors, and Prevention. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 375–382.
- Wood, A.M.; Stockley, R.A. The genetics of chronic obstructive pulmonary disease. Respir. Res. 2006, 7, 130.
- Cerdá, B.; Soto, C.; Albaladejo, M.D.; Martínez, P.; Sánchez-Gascón, F.; Tomás-Barberán, F.; Espín, J.C. Pomegranate juice supplementation in chronic obstructive pulmonary disease: A 5-week randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2006, 60, 245–253.
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans. J. Nutr. 2007, 137, 2196–2201.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- González-Sarrías, A.; Espín, J.-C.; Tomás-Barberán, F.A.; García-Conesa, M.-T. Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol. Nutr. Food Res. 2009, 53, 686–698.
- Cussotto, S.; Walsh, J.; Golubeva, A.V.; Zhdanov, A.V.; Strain, C.R.; Fouhy, F.; Stanton, C.; Dinan, T.G.; Hyland, N.P.; Clarke, G.; et al. The gut microbiome influences the bioavailability of olanzapine in rats. EBioMedicine 2021, 66, 103307.
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106.
- Romo-Vaquero, M.; García-Villalba, R.; González-Sarrías, A.; Beltrán, D.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Interindividual variability in the human metabolism of ellagic acid: Contribution of Gordonibacter to urolithin production. J. Funct. Foods 2015, 17, 785–791.
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538.
- Husari, A.; Hashem, Y.; Bitar, H.; Dbaibo, G.; Zaatari, G.; Sabban, M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. COPD 2016, 11, 227–237.
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258.
- Moghadami, M. A Narrative Review of Influenza: A Seasonal and Pandemic Disease. Iran. J. Med. Sci. 2017, 42, 2–13.
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758.
- Mehrbod, P.; Abdalla, M.A.; Njoya, E.M.; Ahmed, A.S.; Fotouhi, F.; Farahmand, B.; Gado, D.A.; Tabatabaian, M.; Fasanmi, O.G.; Eloff, J.N.; et al. South African medicinal plant extracts active against influenza A virus. BMC Complement. Altern. Med. 2018, 18, 112.
- Haidari, M.; Ali, M.; Ward Casscells, S.; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136.
- Sundararajan, A.; Ganapathy, R.; Huan, L.; Dunlap, J.R.; Webby, R.J.; Kotwal, G.J.; Sangster, M.Y. Influenza virus variation in susceptibility to inactivation by pomegranate polyphenols is determined by envelope glycoproteins. Antivir. Res. 2010, 88, 1–9.
- Moradi, M.-T.; Karimi, A.; Rafieian-Kopaei, M.; Rabiei-Faradonbeh, M.; Momtaz, H. Pomegranate peel extract inhibits internalization and replication of the influenza virus: An in vitro study. Avicenna J. Phytomed. 2020, 10, 143–151.
- Moradi, M.-T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.-S. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J. Med. Biotechnol. 2019, 11, 285–291.
- Baloch, S.; Baloch, M.A.; Zheng, T.; Pei, X. The Coronavirus Disease 2019 (COVID-19) Pandemic. Tohoku J. Exp. Med. 2020, 250, 271–278.
- Heymann, D.L.; Shindo, N. COVID-19: What is next for public health? Lancet 2020, 395, 542–545.
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734.
- Faheem; Kumar, B.K.; Sekhar, K.V.G.C.; Kunjiappan, S.; Jamalis, J.; Balaña-Fouce, R.; Tekwani, B.L.; Sankaranarayanan, M. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorganic Chem. 2020, 104, 104269.
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorganic Chem. 2021, 114, 105145.
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2021, 476, 1179–1193.
- Saadh, M.J.; Almaaytah, A.M.; Alaraj, M.; Dababneh, M.F.; Sa’adeh, I.; Aldalaen, S.M.; Kharshid, A.M.; Alboghdadly, A.; Hailat, M.; Khaleel, A.; et al. Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3908–3913.
- Hsu, M.-F.; Kuo, C.-J.; Chang, K.-T.; Chang, H.-C.; Chou, C.-C.; Ko, T.-P.; Shr, H.-L.; Chang, G.-G.; Wang, A.H.-J.; Liang, P.-H. Mechanism of the Maturation Process of SARS-CoV 3CL Protease. J. Biol. Chem. 2005, 280, 31257–31266.
- Kim, Y.; Mandadapu, S.R.; Groutas, W.C.; Chang, K.-O. Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antivir. Res. 2013, 97, 161–168.
- Khalifa, I.; Zhu, W.; Mohammed, H.H.H.; Dutta, K.; Li, C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CLpro: An in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020, 44, e13432.
- Khalifa, I.; Nawaz, A.; Sobhy, R.; Althwab, S.A.; Barakat, H. Polyacylated anthocyanins constructively network with catalytic dyad residues of 3CLpro of 2019-nCoV than monomeric anthocyanins: A structural-relationship activity study with 10 anthocyanins using in-silico approaches. J. Mol. Graph. Model. 2020, 100, 107690.
- Dubey, V.K. IIT, BHU to Re-Purpose Approved Drugs from DrugBank Database for Treating COVID-19 by Targeting SARS-CoV-2 Main Protease. Available online: https://dst.gov.in/iit-bhu-re-purpose-approved-drugs-drugbank-database-treating-covid-19-targeting-sars-cov-2-main (accessed on 30 September 2022).
- Alkhatib, A.J. The Use of Fresh Pomegranate Juice in the Treatment of Covid-19: Clinical Case Study. PSM Biol. Res. 2021, 6, 1–4.
- American Cancer Society. Global Cancer Facts & Figures 2020: American Cancer Society; American Cancer Society: Atlanta, GA, USA, 2020.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249.
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18, s41–s46.
- Livshits, Z.; Rao, R.B.; Smith, S.W. An Approach to Chemotherapy-Associated Toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Berköz, M.; Krośniak, M. Punicalagin induces apoptosis in A549 cell line through mitochondria-mediated pathway. Genom. Proteom. Bioinform. 2020, 39, 557–567.
- Aqil, F.; Munagala, R.; Vadhanam, M.V.; Kausar, H.; Jeyabalan, J.; Schultz, D.J.; Gupta, R.C. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 2012, 49, 345–353.
- Seeram, N.; Adams, L.; Henning, S.; Niu, Y.; Zhang, Y.; Nair, M.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367.
- Fang, L.; Wang, H.; Zhang, J.; Fang, X. Punicalagin induces ROS-mediated apoptotic cell death through inhibiting STAT3 translocation in lung cancer A549 cells. J. Biochem. Mol. Toxicol. 2021, 35, 1–10.
- Modaeinama, S.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti Tumoral Properties of Punica Granatum (Pomegranate) Peel Extract on Different Human Cancer Cells. Asian Pac. J. Cancer Prev. 2015, 16, 5697–5701.
- Khan, N.; Hadi, N.; Afaq, F.; Syed, D.N.; Kweon, M.-H.; Mukhtar, H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis 2007, 28, 163–173.
- Li, Y.; Yang, F.; Zheng, W.; Hu, M.; Wang, J.; Ma, S.; Deng, Y.; Luo, Y.; Ye, T.; Yin, W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed. Pharmacother. 2016, 80, 227–235.
- Cheng, F.; Dou, J.; Zhang, Y.; Wang, X.; Wei, H.; Zhang, Z.; Cao, Y.; Wu, Z. Urolithin A Inhibits Epithelial–Mesenchymal Transition in Lung Cancer Cells via P53-Mdm2-Snail Pathway. OTT 2021, 14, 3199–3208.
- Husari, A.; Hashem, Y.; Zaatari, G.; El Sabban, M. Pomegranate Juice Prevents the Formation of Lung Nodules Secondary to Chronic Cigarette Smoke Exposure in an Animal Model. Oxidative Med. Cell. Longev. 2017, 2017, 6063201.
This entry is offline, you can click here to edit this entry!
