Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer is the second leading cause of death worldwide after cardiovascular diseases. One of the most promising targeted therapies for cancer treatment is antibody therapy. It has a superior targeting ability for antigens that are expressed on cancer cells, which results in prominent antitumor activity and lower toxicity, compared with that of chemotherapeutic agents. Recent progress in recombinant DNA technology and antibody engineering has ushered in a new era of bispecific antibody (bsAb)-based immune-cell engagers (ICEs), including T- and natural-killer-cell engagers.
- bispecific antibody
- immune-cell engager
- cancer
- therapeutic target
- T-cell
- NK cell
1. Introduction
Cancer is one of the major leading causes of death worldwide. In 2020, nearly 19.3 million new cancer cases and 10.0 million cancer deaths were reported globally. More specifically, the most common cancers among new cases were breast cancer (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers [1]. The order of the mortality rate on the basis of cancer types was as follows: lung (18%), colorectal (9.4%), liver (8.3%), stomach (7.7%), and breast (6.9%) cancers [2]. By 2040, the number of new cancer cases is expected to be approximately 28.3 million, which is nearly >50% from that reported in 2020 [3]. Currently, various therapeutic regimens, such as surgical resection, chemotherapy, antibody therapy, radiotherapy, and combination therapy, have been used in clinical practice for the effective treatment of patients with cancers, depending on their health conditions and cancer status [4].
One of the most promising targeted therapies for cancer treatment is antibody therapy. It has a superior targeting ability for antigens that are expressed on cancer cells, which results in prominent antitumor activity and lower toxicity, compared with that of chemotherapeutic agents [5]. As of December 2021, 110 therapeutic antibodies, including monoclonal antibodies (mAbs), bispecific antibodies (bsAbs), and antibody–drug conjugates (ADCs), have been approved by the United States Food and Drug Administration (US FDA) and/or the European Medicines Agency (EMA). Among them, 46 antibodies are indicated for cancer treatment [6]. Generally, immunoglobulin (Ig) G-based mAb—the most widely used mAb form for antibody therapy—comprises two heavy and two light chains. The light chain has one variable (VL) and one constant (CL) domain, whereas the heavy chain has one variable (VH) and three constant (CH; CH1–CH3) domains [7][8]. Furthermore, the fragment antigen-binding (Fab) region of mAb plays a key role in cancer therapy, and specifically in modulating or blocking the signaling pathways that are involved in cancer development; on the other hand, the fragment crystallizable (Fc) region interacts with Fc receptors that are expressed on immune cells and participates in various effector functions, such as killing cancer cells via antigen-dependent T-cellular cytotoxicity (ADCC) [9][10].
BsAbs harness the specificities of two antibodies and combine them to simultaneously recognize two independent epitopes or antigens [11]. More specifically, these antibodies are designed and manufactured to contain two target-binding units in one antibody-based molecule, whereby each unit independently recognizes its unique epitope, through quadromas, chemical conjugation, or genetic recombination [12]. Compared with mAbs in cancer therapy, bsAbs have several potential benefits, such as the improvement in therapeutic efficacy, the enhancement of tumor-cell selectivity, and the reduction in tumor-cell resistance [13]. In 2009, catumaxomab—a rat–mouse hybrid bsAb for the CD3 epithelial cell adhesion molecule (EpCAM)—was first approved by the EMA for the treatment of malignant ascites in patients with EpCAM-positive cancer [14]. However, it was voluntarily withdrawn from the US market in 2013, and from the European Union (EU) market in 2017, because of commercial reasons [15]. Since the US FDA approval of blinatumomab—a CD19 × CD3 mouse bispecific T-cell engager (BiTE) antibody—for the treatment of acute lymphoblastic leukemia (ALL) in 2014, much attention has been paid to the development of bsAb-based immune-cell engagers (ICEs) that redirect immune effector cells against cancer cells and promote antitumor activities [16][17]. Furthermore, compared with adoptive immune-cell therapy, which requires an expensive and complicated manufacturing process, ICE has become a feasible therapeutic cancer-therapy approach. Currently, increasing numbers of bsAb-based ICEs are extensively evaluated in clinical trials worldwide [18][19].
2. Action Mechanism of bsAb-Based ICEs in Cancer
BsAb-based ICEs play a key role in cancer immunotherapy, and specifically in recruiting and engaging immune effector cells that are proximal to tumor-associated antigens (TAA) that are expressed on cancer cells and that allow the formation of immune synapses and a specialized cell–cell junction between the immune and cancer cells [20][21]. Ultimately, these immune synapses promote the elimination of target cancer cells [22]. These bsAb-based ICEs are currently classified into T- and natural killer (NK)-cell engagers (Figure 1).
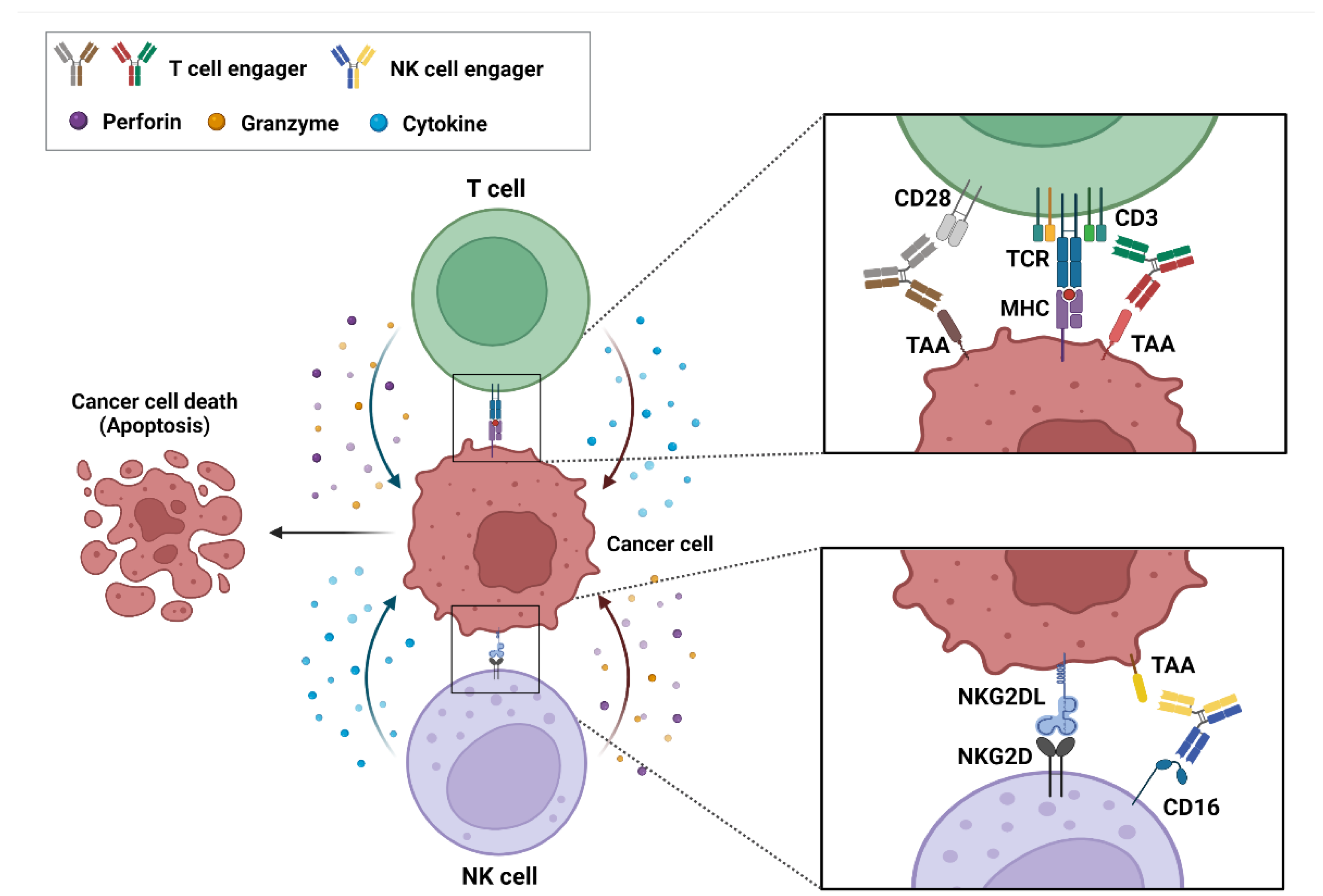
Figure 1. Action mechanism of bispecific antibody (bsAb)-based immune-cell engagers (ICEs) in cancers. The schematic drawing represents bsAb-based ICEs, including T- and natural killer-cell engagers, that bind simultaneously to tumor-associated antigens on cancer cells and specific antigens, such as CD3, CD28, and CD16 on immune cells. These interactions result in the formation of an immune synapse and the activation of immune cells that release cytokines, perforins, and granzymes to induce the cytotoxic effects on cancer cells.
3. Role of Known and Emerging Targets of ICEs
3.1. Single-Pass ICE Targets in Solid Cancers
3.1.1. Delta-Like Ligand 3
Delta-like ligand 3 (DLL3) is a 65-kDa type I transmembrane protein and a Notch receptor ligand. It plays an important role in the regulation of Notch signaling [23]. In small-cell lung cancer (SCLC), DLL3 has been reported as a key factor in the promotion of the tumor growth, migration, and invasion of SCLC cells. Several lines of evidence support this notion [23][24]. Upregulated DLL3 expression was verified to promote tumor growth in a mouse xenograft model that was implanted with DLL3-overexpressing SBC-5 human SCLC cells. Additionally, DLL3 knockdown reduces SCLC-cell migration and invasion, whereas its overexpression in the cells increases these activities [24]. This protein is highly upregulated and aberrantly expressed in SCLC and other neuroendocrine malignancies, but not in nonmalignant T-cells [23][25]. Currently, DLL3 is considered an attractive novel potential therapeutic target in neuroendocrine tumors (NETs), including SCLC. A preclinical study on robalpituzumab tesirin—an ADC that targets DLL3—showed a dose-dependent reduction in the tumor size with a complete response (CR) in B6129SF1/J mice that were implanted with DLL3-positive KP1 SCLC cells, which led to the absence of measurable tumors for >80 days after treatment [26].
3.1.2. Epidermal Growth Factor Receptor
Epidermal growth factor receptor (EGFR) is a 170-kDa receptor tyrosine kinase that belongs to the ErbB family and that comprises two major functional domains—the extracellular and cytoplasmic domains—and a tyrosine kinase domain that is linked by a single transmembrane region [27][28]. EGF binding to the receptor induces the dimerization of the receptor; triggers the autophosphorylation of cytoplasmic tyrosine residues; and eventually participates in the regulation of cell proliferation, migration, and adhesion [27][29][30][31]. EGFR is overexpressed in various cancers, such as colorectal cancer (CRC), lung cancer, breast cancer, glioblastoma, and head and neck squamous cell carcinoma. EGFR overexpression in CRC has been closely associated with tumor progression and poor prognosis [32][33][34][35][36]. Currently, EGFR is one of the most well-known therapeutic targets in various cancers. Phase II clinical studies on cetuximab—a human/mouse chimeric mAb that targets EGFR in advanced CRC—have demonstrated that the use of cetuximab as monotherapy exerts anticancer effects with approximately 10% partial response (PR) and 33% stable disease (SD) [37].
3.1.3. EpCAM
EpCAM is a 40-kDa type I transmembrane glycoprotein that plays a key role in the regulation of cell adhesion, proliferation, and differentiation [38][39][40]. EpCAM is overexpressed in various cancers, such as ovarian cancer, CRC, breast cancer, lung cancer, and pancreatic cancer [41][42][43][44]. Its protease-cleaved intracellular domain associates with β-catenin to form a nuclear protein complex that is translocated to the nucleus, activates the transcription of genes that are involved in cancer-cell proliferation, and results in tumorigenesis [38]. Several studies have suggested EpCAM as a potential target for antibody therapy against cancers. For instance, a phase II clinical study on adecatumumab (MT201)—a fully human mAb that targets EpCAM in metastatic breast cancer—reports that, of 112 patients treated with adecatumumab, 2 showed a PR and 10 had SD, according to the response evaluation criteria in solid tumors (RECIST) [45].
3.1.4. Glycoprotein A33
Glycoprotein A33 (GPA33)—also known as cell surface A33 antigen—is a 43-kDa cell surface differentiation glycoprotein that belongs to the type I transmembrane protein family [46][47][48]. It is associated with cell–cell adhesion [46]. GPA33 is highly overexpressed in >95% of human CRCs but is not detected in any other tissues [48]. Several studies have indicated GPA33 as a potential target in immunotherapy against CRC. For instance, in vivo studies on KRN330—a human mAb that targets GPA33—have demonstrated its dose-dependent antitumor activities in mouse and rat xenograft models implanted with LS174T human CRC cells [49][50].
3.1.5. Human EGFR 2
Human EGFR 2 (HER2) is a member of the ErbB family and is a 185-kDa single-pass transmembrane receptor. To the best of the researchers' knowledge, direct ligands for HER2 have not been identified yet. HER2 activation is achieved through homo- or heterodimerization with HER2 or other ErbB-family receptor members, including EGFR and HER3 [51][52][53]. It is overexpressed in various cancers, such as breast, gastric/gastroesophageal, and colon cancers [54][55][56]. HER2 is closely associated with cancer-cell proliferation and invasion, as well as with tumor growth [57][58]. Particularly in breast cancers, HER2 is overexpressed in 15–30% of the total patients with breast cancers [51]. Substantial evidence has shown that HER2 is an important predictive biomarker in HER2-targeted therapies, and a well-known therapeutic target in breast cancers. Trastuzumab is the first anti-HER2 humanized mAb that targets HER2 in breast cancer. In a phase III clinical study, patients with breast cancer treated with trastuzumab combined with chemotherapy had a longer survival (median survival, 25.1 vs. 20.3 months) and prolonged disease progression (median, 7.4 vs. 4.6 months) than those treated with chemotherapy alone [59].
3.1.6. Mucin 16
Mucin 16 (MUC16)—also known as human carbohydrate antigen 125 (CA-125)—is a heavily glycosylated 300–500-kDa type I transmembrane protein [60][61][62]. It is a biomarker for ovarian cancer. MUC16 is overexpressed in ovarian cancer and contributes to ovarian-cancer progression and metastasis [62][63]. Increased MUC16 expression is associated with poor prognosis in patients with ovarian cancer [64]. Some studies have reported that MUC16 is a potential target for antibody therapy against ovarian cancers. Oregovomab is a mouse mAb that targets MUC16 in advanced ovarian cancer. In a phase II clinical study, 145 patients with stage III/IV ovarian cancer were randomized to receive oregovomab (n = 73) or placebo (n = 72). The time to recurrence was prolonged in the oregovomab group (24.0 months) compared with that in the placebo group (10.8 months) [65].
3.1.7. Mucin 17
Mucin 17 (MUC17) is a 452-kDa type I membrane-associated mucin that is expressed on the apical surface of gastrointestinal epithelial cells [66][67][68]. As a key component of the mucosal layer, MUC17 has been suggested to play a crucial role in the restoration and protection of epithelial cells [66]. Recent studies have demonstrated that aberrant MUC17 overexpression is correlated with the malignant potential of gastric and pancreatic cancers [67][69]. Particularly in gastric-cancer tissues, MUC17 is overexpressed in approximately 50% of the gastric-cancer cases. Thus, MUC17 is a compelling target in gastric cancer because of its prevalent expression on tumor cells compared with its low, relatively restricted expression in normal tissues [70].
3.1.8. Prostate-Specific Membrane Antigen
Prostate-specific membrane antigen (PSMA) is a 100-kDa type II membrane protein, is exclusively overexpressed in prostate cancer, and acts as a glutamate-preferring carboxypeptidase. Its expression is associated with tumor invasiveness [71][72]. PSMA is not only a well-known biomarker but is also a potential therapeutic target in prostate cancer. Its expression is 100–1000-fold higher in prostate-cancer tissue than in normal tissue, and it is present on the cell surface without being released into the circulation [73][74][75][76]. Furthermore, J591 was recently developed as the first humanized mAb that targets the extracellular domain of PSMA in prostate cancer. A phase I/II study was conducted to evaluate the safety and efficacy of J591 in patients with metastatic castration-resistant prostate cancer (mCRPC). Of the 23 patients with measurable disease, 14 (60.8%) had SD and 6 (26.1%) had progressive disease, according to the RECIST [77].
3.2. Multi-Pass Transmembrane Proteins as ICE Targets in Solid Cancers
3.2.1. Claudin-18 Isoform 2
Claudin-18 isoform 2 (CLDN18.2) is a 23-kDa tetra-transmembrane protein. It plays an important role in the regulation of tight junction formation and cell adhesion [78][79][80]. It is known as a tumor-specific marker in gastric or gastroesophageal junction (GEJ) cancers because it is overexpressed exclusively in primary gastric malignancies, but not in any healthy tissues, except stomach mucosa [76][78][81]. Some studies have suggested that CLDN18.2 is a target for antibody therapy against cancer. Claudiximab (IMAB362) is a chimeric mAb that targets CLDN18.2 in gastric cancer. In a phase II study, patients with advanced/recurrent gastric and GEJ cancers who were treated with claudiximab combined with chemotherapy exhibited a significantly improved progression-free survival (PFS) (median, 7.9 vs. 4.8 months) and prolonged overall survival (OS) (median, 13.3 vs. 8.4 months) compared with those treated with chemotherapy alone [82].
3.2.2. Six-Transmembrane Epithelial Antigen of Prostate 1
Six-transmembrane epithelial antigen of prostate 1 (STEAP1) is a 39-kDa integral membrane protein that comprises six transmembrane helices [83][84]. In normal cells, STEAP1 plays a key role in the regulation of cell migration and proliferation, despite its low expression or absence in normal tissues [83][85]. It is highly overexpressed in various cancers, and particularly in prostate cancer, wherein it is involved in the regulation of various functions, such as cancer-cell invasion and proliferation, as well as tumorigenesis [84][85]. The knockdown of STEAP1 has been shown to inhibit T-cell growth in androgen-dependent prostate cancer [86]. Moreover, high STEAP1 expression is closely associated with poor outcomes in patients with prostate cancer [87]. These properties make STEAP1 a potential target for antibody therapy. DSTP3086S—an ADC that targets STEAP1—exhibited antitumor activity in a phase I clinical trial in patients with mCRPC. Of the 46 patients, 2 (4%) showed a PR and 24 (52%) had SD, according to the RECIST [88].
3.2.3. Somatostatin Receptor 2
Somatostatin receptor 2 (SSTR2) is a 41-kDa G protein-coupled receptor (GPCR), which is also known as a seven-transmembrane receptor. It is highly overexpressed in most NETs [89][90][91]. Among the NETs, and particularly in SCLC, the high expression of SSTR2 is closely associated with poor prognosis. Furthermore, the loss of SSTR2 reduced tumor growth in a mouse xenograft model implanted with H1048 human SCLC cells [90]. Several studies have suggested that SSTR2 is a therapeutic target in NETs. For instance, the antitumor efficacy of ADC that targets SSTR2 was evaluated in a mouse xenograft model implanted with BON-1 human NET cells, in which it reduced tumor growth [92].
3.3. Glycosylphosphatidylinositol-Anchored Proteins as ICE Targets in Solid Cancers
3.3.1. Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) is a 180–200-kDa member of the immunoglobulin supergene family. It plays a key role in the regulation of various cellular functions, such as cell interaction, cell adhesion, and immune response [93][94][95]. CEA is one of the most widely used tumor-marker proteins for various cancers, such as colorectal, gastric, and liver cancers [96][97][98]. It is highly overexpressed in 90% of the total CRC cases, and it is closely associated with poor prognosis in patients with CRC [99]. In CRCs, CEA is involved in cancer progression and metastasis, as well as drug resistance [96][100][101][102][103]. Furthermore, CEA appears to be a potential target for antibody therapy against CRC. A preclinical study on IMMU-130—an ADC that targets CEA—revealed that the ADC efficiently reduced tumor growth in a mouse xenograft model implanted with LS174T human CRC cells [104][105].
3.3.2. Glypican 3
Glypican 3 (GPC3) is a 60-kDa glycosylphosphatidylinositol (GPI)-anchored membrane-bound heparin sulfate proteoglycan. It plays an important role in normal cell growth [106][107]. GPC3 is overexpressed in various cancers, such as hepatocellular carcinoma (HCC), lung squamous cell carcinoma, and ovarian clear cell carcinoma [108][109][110]. In particular, it is highly overexpressed in 70–81% of HCCs; its overexpression correlates with the poor prognosis of patients with HCC [107]. Several studies have suggested that GPC3 is a target for antibody therapy against HCCs. For instance, a preclinical study on GC33—a mAb that targets GPC3—demonstrated its prominent antitumor activity in a mouse xenograft mouse model implanted with SK-HEP-1 human HCCs. The administration of 1 mg/kg of GC33 significantly inhibited tumor growth, and that of 5 mg/kg resulted in tumor remission [111].
3.4. Sphingolipid as ICE Targets in Solid Cancers
GD2
GD2 is a 1.6-kDa glycosylated lipid molecule that belongs to the class of glycosphingolipids [112][113][114][115]. It plays a key role in the attachment of tumor cells to extracellular matrix proteins. It is overexpressed in various cancers, such as neuroblastoma, melanoma, and SCLC, but not in normal tissues [113][116][117][118]. Particularly in SCLC and neuroblastoma, GD2 overexpression is involved in cell proliferation [116]. GD2 has been suggested as a target for antibody therapy against cancer. A phase II clinical study on 3F8—a mouse mAb that targets GD2 in patients with neuroblastoma—revealed that, of 16 patients, 1 showed a CR, and 1 showed a mixed response [119].
3.5. Single Transmembrane Proteins as ICE Targets in Hematological Cancers
3.5.1. B-Cell Maturation Antigen
B-cell maturation antigen (BCMA)—a member of tumor necrosis factor receptor superfamily member 17 (TNFRSF17)—is a 20-kDa type III transmembrane protein [120]. BCMA binds to its ligands, such as proliferation-inducing ligand and B-cell-activating factor, which thus promotes the survival of B-cells [121][122]. It is overexpressed in malignant plasma cells, including multiple myeloma (MM) cells, and it plays a crucial role in the growth of MM [123][124]. A preclinical study that was conducted that used a mouse xenograft model implanted with RPMI 8226 human MM cells has shown that BCMA overexpression promotes tumor growth [123]. Furthermore, high BCMA expression is associated with poor prognosis in patients with MM [125]. Substantial evidence has shown that BCMA is a target for antibody therapy against MM. Preclinical studies on belantamab mafodotin—a US FDA-approved ADC that targets BCMA—showed that it efficiently inhibited tumor growth and prolonged survival in a mouse xenograft model implanted with H929 human MM cells [126][127].
3.5.2. CD19
CD19 is a 95-kDa type I transmembrane protein [128]. It is a coreceptor of B-cell antigen receptor (BCR), and it plays a role in regulating B-cell growth [129][130]. It is overexpressed in most B-cell malignancies, such as ALL, non-Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL). The overexpression of CD19 promotes the proliferation and survival of these B-cell malignancies [131][132]. Previous studies have suggested that CD19 is an attractive target for antibody therapy against B-cell malignancies. In a phase IIa clinical study on XmAb5574 (MOR00208)—a humanized mAb that targets CD19—of the total patients with relapsed and/or refractory (R/R) NHL who received XmAb5574 monotherapy, 8% showed a CR [133].
3.5.3. CD22
CD22 is a 140-kDa type I transmembrane protein and an inhibitory coreceptor of the BCR that regulates the overstimulation of B-cells [134][135]. CD22 is overexpressed in various B-cell lymphomas, such as CLL, ALL, and NHL, but it is expressed at low levels on immature B-cells and plasma cells [136][137]. Owing to the restricted expression on the B-cell and the inhibitory function of CD22, CD22 has been indicated as a therapeutic target in B-cell lymphoma [138]. Epratuzumab—a humanized mAb that targets CD22—has been reported to be a CD22 agonistic antibody that leads to B-cell inhibition [139]. In a phase II clinical trial, patients with R/R indolent or aggressive NHL were enrolled to receive epratuzumab combined with rituximab, which is a US FDA-approved anti-CD20 mAb. Of the 16 patients with indolent NHL, 9 showed a CR and an unconfirmed CR, and 1 showed a PR. Furthermore, of the six patients with aggressive NHL, three showed a CR, and one showed a PR [140][141].
3.5.4. CD30
CD30 is a 120-kDa type I transmembrane protein that belongs to the tumor necrosis factor receptor family [142]. It plays a key role in lymphocyte activation and proliferation through the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase pathways that have antiapoptotic and prosurvival benefits [143][144]. It is overexpressed in hematopoietic malignancies, including Hodgkin lymphoma (HL) and NHL, and is associated with the survival of these cells [145][146]. Several studies have suggested that CD30 is a target for antibody therapy against hematologic malignancies. For instance, in vivo studies on XmAb2513—a humanized mAb that targets CD30—showed a significant reduction in the tumor growth, and enhanced survival was observed in a mouse xenograft model implanted with CD30-expressing L540 human HL cells [147].
3.5.5. CD33
CD33—also known as the sialic acid-binding Ig-like lectin 3 (Siglec-3)—is a 67-kDa type I transmembrane protein [148]. It plays a crucial role in the modulation of immune-cell functions, such as phagocytosis, cytokine release, and apoptosis [149][150]. It is overexpressed in acute myeloid lymphoma (AML), and its overexpression is observed in >80% of patients with AML [151]. This increased CD33 expression is correlated with the poor prognosis of patients with AML. In patients with AML who were treated with chemotherapy, the OS rate has been reported to be 42.9% in patients with high CD33 expression, compared with 67.5% in those with low CD33 expression [152]. CD33 is a target for antibody therapy against AML. Gemtuzumab ozogamicin (Mylotarg)—a US FDA-approved ADC that targets CD33—showed promising clinical efficacy in patients with AML [153]. In a phase II clinical study, patients with AML in the first recurrence received gemtuzumab ozogamicin monotherapy; of the 277 patients, 35 showed a CR, and 36 showed a CR with incomplete platelet recovery [154].
3.5.6. CD38
CD38 is a 45-kDa type II transmembrane protein [155]. It is overexpressed in MM cells but shows a low expression in normal lymphoid and myeloid cells [156]. It participates in MM cell survival and proliferation [157]. Previous studies have elucidated that tumor growth decreased in a mouse xenograft model implanted with CD38-knockout RPMI 8226 human MM cells, compared with those nontargeting cells [158]. CD38 is a potential target for antibody therapy against MM. Daratumumab (Darzalex) is the first US FDA-approved human mAb that targets CD38 in the treatment of patients with R/R MM [159]. In a phase III clinical study, patients with R/R MM received chemotherapy (control group) or chemotherapy combined with daratumumab (daratumumab group); the CR rate was significantly higher in the daratumumab group (19.2%) than in the control group (9.0%) [160].
3.5.7. CD123
CD123—the alpha chain of the interleukin-3 (IL-3) receptor—is a 75-kDa type I transmembrane protein [161]. It is overexpressed in leukemic stem cells but shows low or no expression in normal hematopoietic stem cells [162]. CD123 binds to IL-3, which is a hematopoietic growth factor, which leads to the survival and proliferation of various hematologic cancers, such as AML, ALL, and HL [161][163][164][165]. Particularly in AML, increased CD123 expression is associated with a poor prognosis of patients with AML [166]. Previous studies have suggested that CD123 is a target for antibody therapy against AML. In vivo studies on IMGN632—an ADC that targets CD123—have revealed its antitumor activities in a mouse xenograft model implanted with MOLM-13 human AML cells; the mice received IMGN632 or control ADC, and IMGN632 increased the survival of mice compared with the vehicle treatment [167].
3.5.8. C-Type Lectin Domain Family 12 Member A
C-type lectin domain family 12 member A (CLEC12A)—a myeloid inhibitory receptor—is a 31-kDa type II transmembrane protein. It plays a crucial role in the negative regulation of inflammation [168][169]. CLEC12A is specifically expressed in AML and is observed in approximately 90% of patients with AML, but not in normal hematopoietic stem and progenitor cells [170][171]. It is closely associated with the poor prognosis of patients with AML. Previous studies have shown that CLEC12A-positive AML cells are more resistant to chemotherapy than CLEC12A-negative AML cells [172]. Furthermore, the administration of anti-CLEC12A chimeric mAb showed a significant tumor-growth delay of up to 38% in a mouse xenograft model implanted with HL-60 human AML cells [173]. These observations suggest that CLEC12A is a target for antibody therapy against AML.
3.5.9. FMS-Like Tyrosine Kinase 3
FMS-like tyrosine kinase 3 (FLT3)—a receptor tyrosine kinase—is a140–160-kDa type I transmembrane protein [174]. FLT3 promotes the proliferation and differentiation of hematopoietic cells by binding to its ligand [175][176]. FLT3 is overexpressed in AML, and its mutations have been detected in approximately 30% of patients with AML [177][178]. The mutation of FLT3 causes ligand-independent FLT3 signaling and leads to a poor prognosis of patients with AML [179][180]. FLT3 is a potential target for antibody therapy against AML. LY3012218 (IMC-EB10) is a human mAb that targets FLT3, which prevents FLT3 signaling [181]. In preclinical studies, LY3012218 has shown efficacy in a mouse xenograft model implanted with MOLM-14 human AML cells; LY3012218 exerts its effects by reducing the engraftment of leukemic cells and extending survival [182].
3.6. Multi-Pass Transmembrane Proteins as ICE Targets in Hematological Cancers
3.6.1. CD20
CD20 is a 33–37-kDa tetra-transmembrane protein [183]. It is involved in B-cell differentiation and it is overexpressed in most B-cell malignancies, such as follicular lymphoma (FL), but not in hematopoietic stem cells or plasma cells [184][185][186]. Several studies have shown that CD20 is a potential target for antibody therapy against FL. Rituximab (Rituxan) is a chimeric mAb that has been approved by the US FDA against CD20 [141]. In a phase III clinical study, patients with R/R FL received lenalidomide plus rituximab or placebo plus rituximab; the median PFS increased in the lenalidomide-plus-rituximab group (39.4 months), compared with that in the placebo-plus-rituximab group (14.1 months) [187].
3.6.2. GPCR Class C Group 5 Member D
GPCR class C group 5 member D (GPRC5D) is a 39-kDa seven-transmembrane protein [188]. It is an orphan receptor that is normally expressed only in the hair follicle. GPRC5D is overexpressed in MM and is unlikely to be shed from the membrane, which prevents the decrease in the efficacy of GPRC5D-targeted therapy [189][190][191]. The role of GPRC5D in cancers is yet to be defined; nonetheless, selective GPRC5D expression may be valuable as a target for antibody therapy against MM. Figure 2 summarizes the known and emerging targets for ICE therapy against cancers.
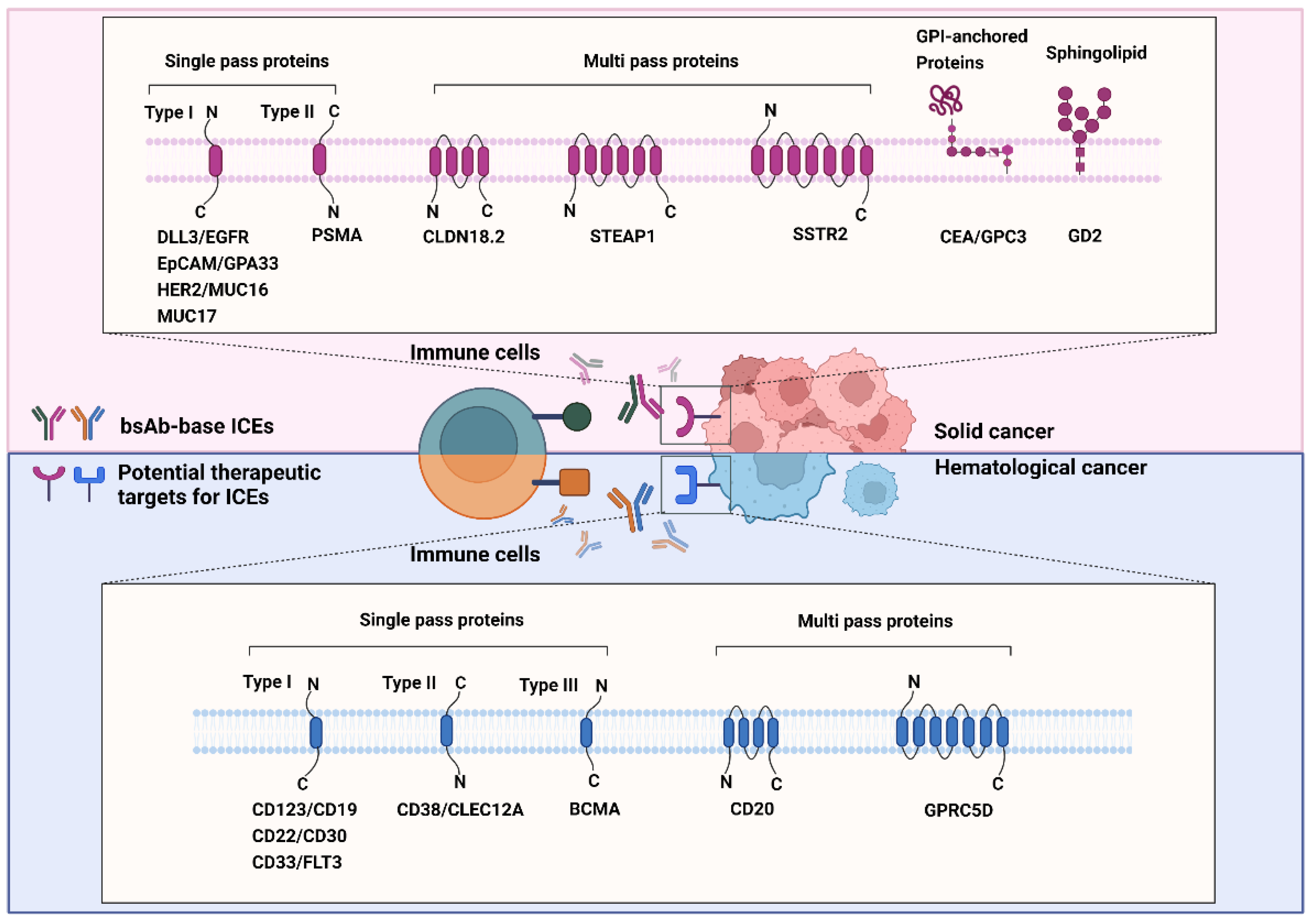
Figure 2. Known and emerging targets for immune-cell engager (ICE) therapy against cancers. The schematic representation shows known and emerging therapeutic targets of bispecific antibody-based ICEs in solid (red background) and hematological (blue background) cancers. All therapeutic targets listed in this figure are grouped on the basis of their relationship with the bilayer and transmembrane topology.
4. Design and Structure of bsAb-Based ICEs
BsAb-based ICEs are designed to contain two different antigen-binding sites that comprise determinants from the VH and VL chains of different antibodies that are specific to each target [192]. Thus far, various efforts have been made to increase their homogeneity, yield, and functional properties to generate desired bsAb-based ICEs [11]. On the basis of the bsAb-based ICE structures, they are divided into two categories: Fv-based ICEs and immunoglobulin G (IgG)-based ICEs (Figure 3). Fv-based ICEs are easy to produce and show lower immunogenicity, whereas IgG-based ICEs have higher solubility, stability, affinity, and extended half-life in serum [193].
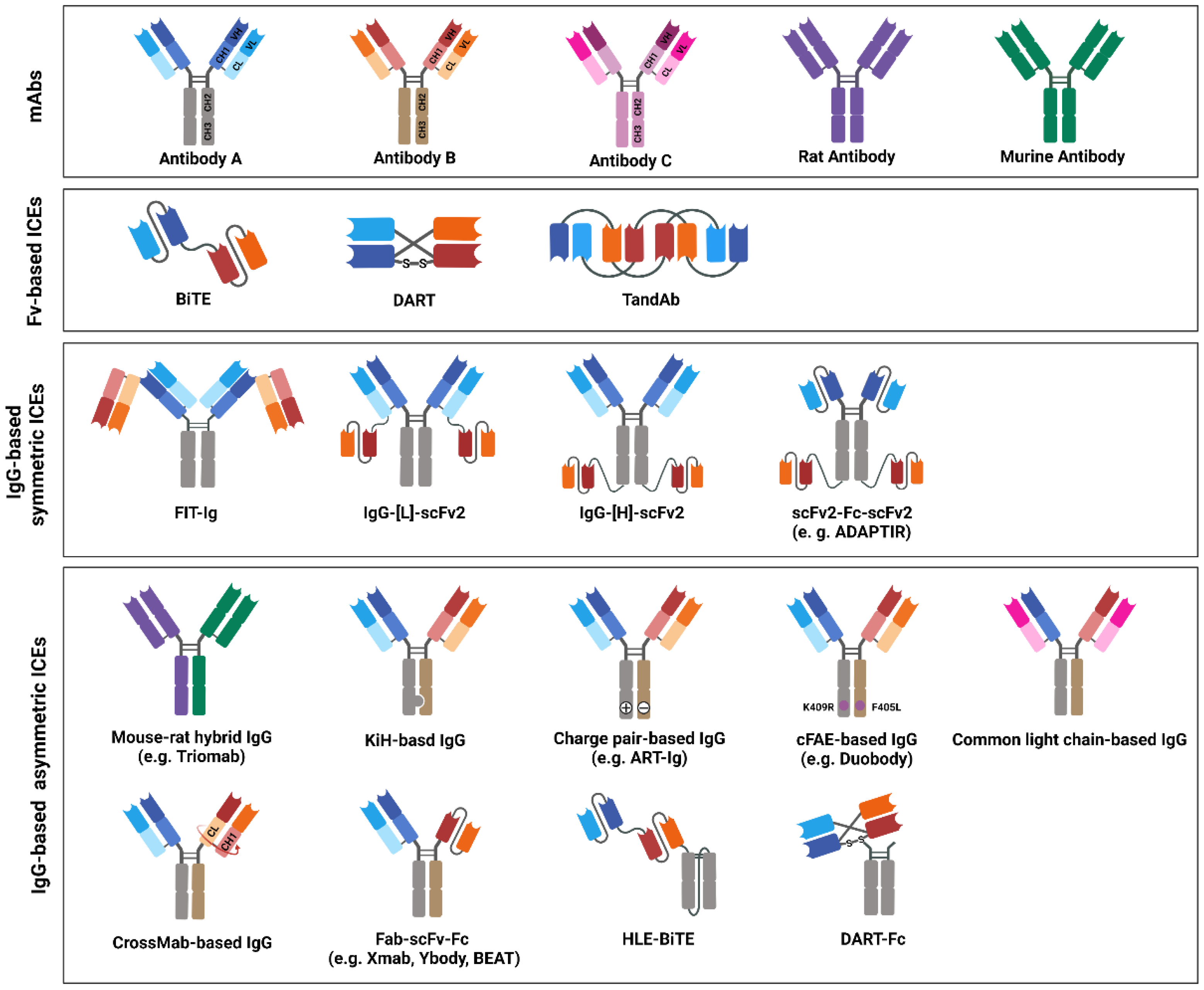
Figure 3. Immune-cell engager (ICE) structures in clinical studies, or ICEs approved by the United States Food and Drug Administration (US FDA) and/or European Medicines Agency (EMA). The schematic drawing depicts the structures of bispecific antibody (bsAb)-based ICEs that are currently evaluated in clinical studies or that have been approved by the US FDA and/or EMA. The structures of bsAb-based ICEs are subdivided into three major classes: variable fragments -based, immunoglobulin G (IgG)-based symmetric, and IgG-based asymmetric ICEs. Variable heavy-chain domains (VHs) of two different antibodies, designated as antibodies A, B, or C, are shown in dark blue, red, or pink, respectively. The variable light-chain domains (VLs) are shown in light blue, red, and pink, respectively. Moreover, rat or mouse antibody is depicted in purple or green, respectively.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23105686
References
- Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysispie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 21 February 2022).
- Estimated Number of Deaths in 2020, Worldwide, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/online-analysispie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 21 February 2022).
- Estimated Number of New Cases From 2020 to 2040, Both Sexes, Ages (0–85). Available online: https://gco.iarc.fr/tomorrow/en/dataviz/bars?types=0&sexes=0&mode=population&group_populations=1&multiple_populations=1&multiple_cancers=1&cancers=39&populations=903_904_905_908_909_935&apc=cat_ca20v1.5_ca23v1.5&group_cancers=1 (accessed on 21 February 2022).
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers 2011, 3, 3279–3330.
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2021, 12, 48.
- Baldo, B.A. Immune- and Non-Immune-Mediated Adverse Effects of Monoclonal Antibody Therapy: A Survey of 110 Approved Antibodies. Antibodies 2022, 11, 17.
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. The structure of a typical antibody molecule. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001.
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520.
- Redman, J.M.; Hill, E.M.; AlDeghaither, D.; Weiner, L.M. Mechanisms of action of therapeutic antibodies for cancer. Mol. Immunol. 2015, 67, 28–45.
- Van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548.
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608.
- Wang, S.; Chen, K.; Lei, Q.; Ma, P.; Yuan, A.Q.; Zhao, Y.; Jiang, Y.; Fang, H.; Xing, S.; Fang, Y. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol. Med. 2021, 13, 14291.
- Mazor, Y.; Oganesyan, V.; Yang, C.; Hansen, A.; Wang, J.; Liu, H.; Sachsenmeier, K.; Carlson, M.; Gadre, D.V.; Borrok, M.J.; et al. Improving target cell specificity using a novel monovalent bispecific IgG design. MAbs 2015, 7, 377–389.
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab: Clinical development and future directions. mAbs 2010, 2, 129–136.
- Ruf, P.; Bauer, H.W.; Schoberth, A.; Kellermann, C.; Lindhofer, H. First time intravesically administered trifunctional antibody catumaxomab in patients with recurrent non-muscle invasive bladder cancer indicates high tolerability and local immunological activity. Cancer Immunol. Immunother. 2021, 70, 2727–2735.
- Nagorsen, D.; Kufer, P.; Baeuerle, P.A.; Bargou, R. Blinatumomab: A historical perspective. Pharmacol. Ther. 2012, 136, 334–342.
- Garber, K. Bispecific antibodies rise again: Amgen’s blinatumomab is setting the stage for a bispecific-antibody revival, enabled by new formats that may solve the field’s long-standing problems. Nat. Rev. Drug Discov. 2014, 13, 799–802.
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38.
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75.
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227.
- Dustin, M.L. The immunological synapse. Cancer Immunol. Res. 2014, 2, 1023–1033.
- Thiery, J.; Keefe, D.; Boulant, S.; Boucrot, E.; Walch, M.; Martinvalet, D.; Goping, I.S.; Bleackley, R.C.; Kirchhausen, T.; Lieberman, J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat. Immunol. 2011, 12, 770–777.
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.D.; Carbone, D.P.; He, K. DLL3: An emerging target in small cell lung cancer. J. Hematol. Oncol. 2019, 12, 61.
- Furuta, M.; Kikuchi, H.; Shoji, T.; Takashima, Y.; Kikuchi, E.; Kikuchi, J.; Kinoshita, I.; Dosaka-Akita, H.; Sakakibara-Konishi, J. DLL 3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci. 2019, 110, 1599–1608.
- Furuta, M.; Sakakibara, J.K.; Shoji, T.; Takashima, Y.; Kikuchi, H.; Kikuchi, E.; Kikuchi, J.; Kinoshita, I.; Akita, H.D.; Nishimura, M. Abstract 3158: DLL3 regulates migration and invasion of small cell lung cancer. Cancer Res. 2018, 78, 3158.
- Vitorino, P.; Chuang, C.-H.; Iannello, A.; Zhao, X.; Anderson, W.; Ferrando, R.; Zhang, Z.; Madhavan, S.; Karsunky, H.; Saunders, L. Rova-T enhances the anti-tumor activity of anti-PD1 in a murine model of small cell lung cancer with endogenous Dll3 expression. Transl. Oncol. 2021, 14, 100883.
- Ribeiro, F.A.P.; Noguti, J.; Oshima, C.T.F.; Ribeiro, D.A. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: A promising approach. Anticancer. Res. 2014, 34, 1547–1552.
- Gschwind, A.; Fischer, O.M.; Ullrich, A. The discovery of receptor tyrosine kinases: Targets for cancer therapy. Nat. Rev. Cancer 2004, 4, 361–370.
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20.
- Yaish, P.; Gazit, A.; Gilon, C.; Levitzki, A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science 1988, 242, 933–935.
- Lu, Z.; Jiang, G.; Blume-Jensen, P.; Hunter, T. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Mol. Cell Biol. 2001, 21, 4016–4031.
- Spano, J.P.; Lagorce, C.; Atlan, D.; Milano, G.; Domont, J.; Benamouzig, R.; Attar, A.; Benichou, J.; Martin, A.; Morere, J.F. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann. Oncol. 2005, 16, 102–108.
- Richard, J.; Sainsbury, C.; Needham, G.; Farndon, J.; Malcolm, A.; Harris, A. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet 1987, 329, 1398–1402.
- Ekstrand, A.J.; James, C.D.; Cavenee, W.K.; Seliger, B.; Pettersson, R.F.; Collins, V.P. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991, 51, 2164–2172.
- Hendler, F.J.; Ozanne, B.W. Human squamous cell lung cancers express increased epidermal growth factor receptors. J. Clin. Investig. 1984, 74, 647–651.
- Ishitoya, J.; Toriyama, M.; Oguchi, N.; Kitamura, K.; Ohshima, M.; Asano, K.; Yamamoto, T. Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Brit. J. Cancer 1989, 59, 559–562.
- You, B.; Chen, E. Anti-EGFR Monoclonal antibodies for treatment of colorectal cancers: Development of cetuximab and panitumumab. J. Clin. Pharmacol. 2012, 52, 128–155.
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases. Int. J. Mol. Med. 2018, 42, 1771–1785.
- Trzpis, M.; McLaughlin, P.M.J.; de Leij, L.M.F.H.; Harmsen, M.C. Epithelial cell adhesion molecule: More than a carcinoma marker and adhesion molecule. Am. J. Pathol. 2007, 171, 386–395.
- Münz, M.; Kieu, C.; Mack, B.; Schmitt, B.; Zeidler, R.; Gires, O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 2004, 23, 5748–5758.
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Brit. J. Cancer 2006, 94, 128–135.
- Kim, J.-H.; Herlyn, D.; Wong, K.-K.; Park, D.-C.; Schorge, J.O.; Lu, K.H.; Skates, S.J.; Cramer, D.W.; Berkowitz, R.S.; Mok, S.C. Identification of Epithelial Cell Adhesion Molecule Autoantibody in Patients with Ovarian Cancer. Clin. Cancer Res. 2003, 9, 4782–4791.
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM Is Overexpressed in Breast Cancer and Is a Potential Target for Breast Cancer Gene Therapy. Cancer Res. 2004, 64, 5818–5824.
- Herlyn, M.; Steplewski, Z.; Herlyn, D.; Koprowski, H. Colorectal carcinoma-specific antigen: Detection by means of monoclonal antibodies. Proc. Natl. Acad. Sci. USA 1979, 76, 1438–1442.
- Schmidt, M.; Scheulen, M.E.; Dittrich, C.; Obrist, P.; Marschner, N.; Dirix, L.; Rüttinger, D.; Schuler, M.; Reinhardt, C.; Awada, A. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann. Oncol. 2010, 21, 275–282.
- Lopes, N.; Bergsland, C.; Bruun, J.; Bjørnslett, M.; Vieira, A.F.; Mesquita, P.; Pinto, R.; Gomes, R.; Cavadas, B.; Bennett, E. A panel of intestinal differentiation markers (CDX2, GPA33, and LI-cadherin) identifies gastric cancer patients with favourable prognosis. Gastric Cancer 2020, 23, 811–823.
- Heath Joan , K.; White Sara , J.; Johnstone, C.N.; Catimel, B.; Simpson, R.J.; Moritz, R.L.; Tu, G.-F.; Ji, H.; Whitehead, R.H.; Groenen, L.C.; et al. The human A33 antigen is a transmembrane glycoprotein and a novel member of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 469–474.
- Wei, D.; Fan, Q.; Cai, H.; Yang, H.; Wan, L.; Li, L.; Lu, X. CF750-A33scFv-Fc-based optical imaging of subcutaneous and orthotopic xenografts of GPA33-positive colorectal cancer in mice. BioMed Res. Int. 2015, 2015, 505183.
- Sawada, N.; Taguchi, E.; Takahashi, M. In vitro and in vivo activities of KRN330, a fully human monoclonal antibody against colon cancer. J. Clin. Oncol. 2011, 29, 432.
- Berlin, J.D.; Infante, J.R.; Taguchi, E.; Goff, L.W.; Jones, S.F.; Chan, E.; Bendell, J.C.; Rothenberg, M.L.; Burris, H.A. In vivo antibody binding to tumor in xenograft rodent models and colorectal cancer patients treated with anti-A33 antibody KRN330. Cancer Res. 2010, 70, 2431.
- Iqbal, N.; Iqbal, N.J.M. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014, 2014, 852748.
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996, 16, 5276–5287.
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655.
- SiShi, L.; Buchbinder, E.; Wu, L.; Bjorge, J.D.; Fujita, D.J.; Zhu, S. EGFR and HER2 levels are frequently elevated in colon cancer cells. Discov. Rep. 2014, 1, 1.
- Lemoine, N.R.; Jain, S.; Silvestre, F.; Lopes, C.; Hughes, C.M.; McLelland, E.; Gullick, W.J.; Filipe, M.I. Amplification and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Brit. J. Cancer 1991, 64, 79.
- Oh, J.J.; Grosshans, D.R.; Wong, S.G.; Slamon, D.J. Identification of differentially expressed genes associated with HER-2/neu overexpression in human breast cancer cells. Nucleic Acids Res. 1999, 27, 4008–4017.
- Tai, W.; Qin, B.; Cheng, K. Inhibition of breast cancer cell growth and invasiveness by dual silencing of HER-2 and VEGF. Mol. Pharm. 2010, 7, 543–556.
- Roh, H.; Pippin, J.; Drebin, J.A. Down-regulation of HER2/neu expression induces apoptosis in human cancer cells that overexpress HER2/neu. Cancer Res. 2000, 60, 560–565.
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792.
- Haridas, D.; Ponnusamy, M.P.; Chugh, S.; Lakshmanan, I.; Seshacharyulu, P.; Batra, S.K. MUC16: Molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014, 28, 4183–4199.
- Aithal, A.; Rauth, S.; Kshirsagar, P.; Shah, A.; Lakshmanan, I.; Junker, W.M.; Jain, M.; Ponnusamy, M.P.; Batra, S.K. MUC16 as a novel target for cancer therapy. Expert Opin. Tar. 2018, 22, 675–686.
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129.
- Comamala, M.; Pinard, M.; Thériault, C.; Matte, I.; Albert, A.; Boivin, M.; Beaudin, J.; Piché, A.; Rancourt, C. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Brit. J. Cancer 2011, 104, 989–999.
- Liu, J.; Li, L.; Luo, N.; Liu, Q.; Liu, L.; Chen, D.; Cheng, Z.; Xi, X.J.E.; Medicine, T. Inflammatory signals induce MUC16 expression in ovarian cancer cells via NF-κB activation. Exp. Ther. Med. 2021, 21, 1.
- Berek, J.S.; Taylor, P.T.; Gordon, A.; Cunningham, M.J.; Finkler, N.; Orr, J., Jr.; Rivkin, S.; Schultes, B.C.; Whiteside, T.L.; Nicodemus, C.F. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J. Clin. Oncol. 2004, 22, 3507–3516.
- Yang, B.; Wu, A.W.; Hu, Y.Q.; Tao, C.J.; Wang, J.M.; Lu, Y.Y.; Xing, R. Mucin 17 inhibits the progression of human gastric cancer by limiting inflammatory responses through a MYH9-p53-RhoA regulatory feedback loop. J. Exp. Clin. Canc. Res. 2019, 38, 283.
- Lordick, F.; Orra, E.B.; Cervantes, A.; Dayyani, F.; Rocha-Lima, C.; Greil, R.; van Laarhoven, H.; Lorenzen, S.; Kischel, R.; Shitara, K. P-76 A phase 1 study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE®) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction cancer. Ann. Oncol. 2020, 31, S114.
- Panwar, H.; Rokana, N.; Aparna, S.V.; Kaur, J.; Singh, A.; Singh, J.; Singh, K.S.; Chaudhary, V.; Puniya, A.K. Gastrointestinal stress as innate defence against microbial attack. J. Appl. Microbiol. 2021, 130, 1035–1061.
- Junker, W.M. Molecular and Biological Studies of MUC17; University of Nebraska Medical Center: Omaha, NE, USA, 2008.
- MUC17. Available online: https://www.amgenoncology.com/targets/MUC17.html (accessed on 14 April 2022).
- Chang, S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004, 6, S13.
- Wang, F.; Li, Z.; Feng, X.; Yang, D.; Lin, M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2021, 25, 1–16.
- Oh, S.W.; Cheon, G.J. Prostate-specific membrane antigen PET imaging in prostate cancer: Opportunities and challenges. Korean J. Radiol. 2018, 19, 819–831.
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma. Cancer 1998, 82, 2256–2261.
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.G.; Kaufman, R.P., Jr.; Kaur, P.; Gray, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakury, B.V.S. Correlation of Primary Tumor Prostate-Specific Membrane Antigen Expression with Disease Recurrence in Prostate Cancer. Clin. Cancer Res. 2003, 9, 6357–6362.
- Trover, J.K.; Beckett, M.L.; Wright, G.L., Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer 1995, 62, 552–558.
- Tagawa, S.T.; Vallabhajosula, S.; Christos, P.J.; Jhanwar, Y.S.; Batra, J.S.; Lam, L.; Osborne, J.; Beltran, H.; Molina, A.M.; Goldsmith, S.J.J.C. Phase 1/2 study of fractionated dose lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 2019, 125, 2561–2569.
- Baek, J.H.; Park, D.J.; Kim, G.Y.; Cheon, J.; Kang, B.W.; Cha, H.J.; Kim, J. Clinical implications of Claudin18. 2 expression in patients with gastric cancer. Anticancer. Res. 2019, 39, 6973–6979.
- Hashimoto, Y.; Yagi, K.; Kondoh, M. Current progress in a second-generation claudin binder, anti-claudin antibody, for clinical applications. Drug Discov. Today 2016, 21, 1711–1718.
- Lin, H.; Zhang, R. Abstract B73: Development of anti-human CLDN18. 2 monoclonal antibody as cancer therapeutics. Cancer Immunol. Res. 2020, 8, B73.
- Sanada, Y.; Oue, N.; Mitani, Y.; Yoshida, K.; Nakayama, H.; Yasui, W. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J. Pathol. 2006, 208, 633–642.
- Singh, P.; Toom, S.; Huang, Y.J. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J. Hematol. Oncol. 2017, 10, 105.
- Barroca-Ferreira, J.; Cruz-Vicente, P.; Santos, M.F.; Rocha, S.M.; Santos-Silva, T.; Maia, C.J.; Passarinha, L.A. Enhanced Stability of Detergent-Free Human Native STEAP1 Protein from Neoplastic Prostate Cancer Cells upon an Innovative Isolation Procedure. Int. J. Mol. Sci. 2021, 22, 10012.
- Hubert Rene, S.; Vivanco, I.; Chen, E.; Rastegar, S.; Leong, K.; Mitchell Steve, C.; Madraswala, R.; Zhou, Y.; Kuo, J.; Raitano Arthur, B.; et al. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 14523–14528.
- Wu, Y.-Y.; Jiang, J.-N.; Fang, X.-D.; Ji, F.-J.J. STEAP1 regulates tumorigenesis and chemoresistance during peritoneal metastasis of gastric cancer. Front. Physiol. 2018, 9, 1132.
- Chen, W.-J.; Wu, H.-T.; Li, C.-L.; Lin, Y.-K.; Fang, Z.-X.; Lin, W.-T.; Liu, J. Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Front. Cell Dev. Biol. 2021, 9, 2988.
- Rocha, S.; Barroca-Ferreira, J.; Passarinha, L.; Socorro, S.; Maia, C.J.E.P. The Usefulness of STEAP Proteins in Prostate Cancer Clinical Practice. Exon Publ. 2021, 10, 139–153.
- Activity of Anti-STEAP1 Antibody-Drug Conjugate in Patients With Metastatic Castration-Resistant Prostate Cancer. Available online: https://ascopost.com/news/november-2019/activity-of-anti-steap1-antibody-drug-conjugate-in-patients-with-mcrpc/ (accessed on 30 March 2022).
- Lehman, J.; Hoeksema, M.; Chen, H.; Shi, C.; Eisenberg, R.; Massion, P.P. Loss of somatostatin receptor 2 expression and lung cancer growth. J. Clin. Oncol. 2015, 33, 7569.
- Lehman, J.M.; Hoeksema, M.D.; Staub, J.; Qian, J.; Harris, B.; Callison, J.C.; Miao, J.; Shi, C.; Eisenberg, R.; Chen, H. Somatostatin receptor 2 signaling promotes growth and tumor survival in small-cell lung cancer. Int. J. Cancer 2019, 144, 1104–1114.
- Reisine, T.; Bell, G.I. Molecular biology of somatostatin receptors. Endocr. Rev. 1995, 16, 427–442.
- Si, Y.; Kim, S.; Ou, J.; Lu, Y.; Ernst, P.; Chen, K.; Whitt, J.; Carter, A.M.; Markert, J.M.; Bibb, J.A. Anti-SSTR2 antibody-drug conjugate for neuroendocrine tumor therapy. Cancer Gene Ther. 2021, 28, 799–812.
- Kammerer, R.; Zimmermann, W. Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 2010, 8, 12.
- Benchimol, S.; Fuks, A.; Jothy, S.; Beauchemin, N.; Shirota, K.; Stanners, C.P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell 1989, 57, 327–334.
- Oikawa, S.; Inuzuka, C.; Kuroki, M.; Matsuoka, Y.; Kosaki, G.; Nakazato, H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: Homophilic and heterophilic adhesion. Biochem. Biophys. Res. Commun. 1989, 164, 39–45.
- Lee, J.H.; Lee, S.-W. The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches. Gastroenterol. Res. Pract. 2017, 2017, 7521987.
- Deng, K.; Yang, L.; Hu, B.; Wu, H.; Zhu, H.; Tang, C. The Prognostic Significance of Pretreatment Serum CEA Levels in Gastric Cancer: A Meta-Analysis Including 14651 Patients. PLoS ONE 2015, 10, e0124151.
- Pakdel, A.; Malekzadeh, M.; Naghibalhossaini, F. The association between preoperative serum CEA concentrations and synchronous liver metastasis in colorectal cancer patients. Cancer Biomark. 2016, 16, 245–252.
- Auclin, E.; Taieb, J.; Lepage, C.; Aparicio, T.; Faroux, R.; Mini, E.; Folprecht, G.; Salazar, R.; Benetkiewicz, M.; Banzi, M.J.C.E.; et al. Carcinoembryonic antigen levels and survival in stage III colon cancer: Post hoc analysis of the MOSAIC and PETACC-8 trials. Cancer Epidemiol. Prev. Biomark. 2019, 28, 1153–1161.
- Campos-da-Paz, M.; Dórea, J.G.; Galdino, A.S.; Lacava, Z.G.M.; de Fatima, M.A.S. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: Update on biomarker for clinical and biotechnological approaches. Recent Pat. Biotechnol. 2018, 12, 269–279.
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671.
- Thomas, P.; Gangopadhyay, A.; Steele, G., Jr.; Andrews, C.; Nakazato, H.; Oikawa, S.; Jessup, J.M. The effect of transfection of the CEA gene on the metastatic behavior of the human colorectal cancer cell line MIP-101. Cancer Lett. 1995, 92, 59–66.
- Stein, R.; Goldenberg, D.M. A humanized monoclonal antibody to carcinoembryonic antigen, labetuzumab, inhibits tumor growth and sensitizes human medullary thyroid cancer xenografts to dacarbazine chemotherapy. Mol. Cancer Ther. 2004, 3, 1559–1564.
- Segal, N.H.; Verghis, J.; Govindan, S.; Maliakal, P.; Sharkey, R.M.; Wegener, W.A.; Goldenberg, D.M.; Saltz, L.B. Abstract LB-159: A Phase I study of IMMU-130 (labetuzumab-SN38) anti-CEACAM5 antibody-drug conjugate (ADC) in patients with metastatic colorectal cancer (mCRC). Cancer Res. 2013, 73, LB-159.
- Govindan, S.V.; Cardillo, T.M.; Rossi, E.A.; McBride, W.J.; Sharkey, R.M.; Goldenberg, D.M. IMMU-130, a unique antibody-drug conjugate (ADC) of SN-38 targeting CEACAM5 antigen: Preclinical basis for clinical activity in metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2015, 33, 625.
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J. Histochem. Cytochem. 2020, 68, 841–862.
- Ofuji, K.; Saito, K.; Yoshikawa, T.; Nakatsura, T. Critical analysis of the potential of targeting GPC3 in hepatocellular carcinoma. J. Hepatocell. Carcinoma 2014, 1, 35.
- Capurro, M.I.; Xiang, Y.-Y.; Lobe, C.; Filmus, J. Glypican-3 Promotes the Growth of Hepatocellular Carcinoma by Stimulating Canonical Wnt Signaling. Cancer Res. 2005, 65, 6245–6254.
- Aviel-Ronen, S.; Lau, S.K.; Pintilie, M.; Lau, D.; Liu, N.; Tsao, M.S.; Jothy, S. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod. Pathol. 2008, 21, 817–825.
- Maeda, D.; Ota, S.; Takazawa, Y.; Aburatani, H.; Nakagawa, S.; Yano, T.; Taketani, Y.; Kodama, T.; Fukayama, M. Glypican-3 expression in clear cell adenocarcinoma of the ovary. Mod. Pathol. 2009, 22, 824–832.
- Ishiguro, T.; Sugimoto, M.; Kinoshita, Y.; Miyazaki, Y.; Nakano, K.; Tsunoda, H.; Sugo, I.; Ohizumi, I.; Aburatani, H.; Hamakubo, T. Anti–glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008, 68, 9832–9838.
- Doronin, I.I.; Vishnyakova, P.A.; Kholodenko, I.V.; Ponomarev, E.D.; Ryazantsev, D.Y.; Molotkovskaya, I.M.; Kholodenko, R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014, 14, 295.
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 2020, 10, 1000.
- Dobrenkov, K.; Cheung, N.-K.V. GD2-targeted immunotherapy and radioimmunotherapy. Semin. Oncol. 2014, 41, 589–612.
- GD2 Ganglioside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/GD2-Ganglioside (accessed on 15 April 2022).
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside GD2 in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252.
- Navid, F.; Santana, V.M.; Barfield, R.C. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets 2010, 10, 200–209.
- Cheresh, D.A.; Pierschbacher, M.D.; Herzig, M.A.; Mujoo, K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J. Cell Biol. 1986, 102, 688–696.
- Cheung, N.; Kushner, B.H.; Yeh, S.; Larson, S.M. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: A phase II study. Int. J. Oncol. 1998, 12, 1299–1605.
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005.
- Schiemann, B.; Gommerman Jennifer, L.; Vora, K.; Cachero Teresa, G.; Shulga-Morskaya, S.; Dobles, M.; Frew, E.; Scott Martin, L. An Essential Role for BAFF in the Normal Development of B Cells Through a BCMA-Independent Pathway. Science 2001, 293, 2111–2114.
- Yu, G.; Boone, T.; Delaney, J.; Hawkins, N.; Kelley, M.; Ramakrishnan, M.; McCabe, S.; Qiu, W.-R.; Kornuc, M.; Xia, X.-Z.; et al. APRIL and TALL-1 and receptors BCMA and TACI: System for regulating humoral immunity. Nat. Immunol. 2000, 1, 252–256.
- Tai, Y.-T.; Acharya, C.; An, G.; Moschetta, M.; Zhong, M.Y.; Feng, X.; Cea, M.; Cagnetta, A.; Wen, K.; van Eenennaam, H.; et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood 2016, 127, 3225–3236.
- Miao, Y.R.; Cenik, C.; Jiang, D.; Mizuno, K.; Li, G.C.; Zhao, H.; Thakker, K.; Diep, A.; Xu, J.Y.; Zhang, X.E.; et al. Aberrant BCMA Signaling Promotes Tumor Growth by Altering Protein Translation Machinery, a Therapeutic Target for the Treatment of Relapse/Refractory Multiple Myeloma. bioRxiv 2021.
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br. J. Haematol. 2012, 158, 727–738.
- Ketchum, E.B.; Clarke, A.; Clemmons, A.B. Belantamab Mafodotin-blmf: A Novel Antibody-Drug Conjugate for Treatment of Patients With Relapsed/Refractory Multiple Myeloma. J. Adv. Pract. Oncol. 2022, 13, 77–85.
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138.
- Tedder, T.F.; Zhou, L.-J.; Engel, P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol. Today 1994, 15, 437–442.
- Van Zelm, M.C.; Reisli, I.; van der Burg, M.; Castaño, D.; van Noesel, C.J.M.; van Tol, M.J.D.; Woellner, C.; Grimbacher, B.; Patiño, P.J.; van Dongen, J.J.M. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 2006, 354, 1901–1912.
- Carter, R.H.; Fearon, D.T. CD19: Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992, 256, 105–107.
- Anderson, K.C.; Bates, M.P.; Slaughenhoupt, B.L.; Pinkus, G.S.; Schlossman, S.F.; Nadler, L.M. Expression of human B cell-associated antigens on leukemias and lymphomas: A model of human B cell differentiation. Blood 1984, 63, 1424–1433.
- He, X.; Kläsener, K.; Iype, J.M.; Becker, M.; Maity, P.C.; Cavallari, M.; Nielsen, P.J.; Yang, J.; Reth, M. Continuous signaling of CD 79b and CD 19 is required for the fitness of Burkitt lymphoma B cells. EMBO J. 2018, 37, e97980.
- Jurczak, W.; Zinzani, P.L.; Gaidano, G.; Goy, A.; Provencio, M.; Nagy, Z.; Robak, T.; Maddocks, K.; Buske, C.; Ambarkhane, S.; et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann. Oncol. 2018, 29, 1266–1272.
- Walker, J.A.; Smith, K.G.C. CD22: An inhibitory enigma. Immunology 2008, 123, 314–325.
- Nitschke, L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr. Opin. Immunol. 2005, 17, 290–297.
- Shah, N.N.; Stevenson, M.S.; Yuan, C.M.; Richards, K.; Delbrook, C.; Kreitman, R.J.; Pastan, I.; Wayne, A.S. Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatric Blood Cancer 2015, 62, 964–969.
- Jasper, G.A.; Arun, I.; Venzon, D.; Kreitman, R.J.; Wayne, A.S.; Yuan, C.M.; Marti, G.E.; Stetler-Stevenson, M. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytom. Part B Clin. Cytom. 2011, 80B, 83–90.
- Schweizer, A.; Wöhner, M.; Prescher, H.; Brossmer, R.; Nitschke, L. Targeting of CD 22-positive B-cell lymphoma cells by synthetic divalent sialic acid analogues. Eur. J. Immunol. 2012, 42, 2792–2802.
- Ereño-Orbea, J.; Sicard, T.; Cui, H.; Mazhab-Jafari, M.T.; Benlekbir, S.; Guarné, A.; Rubinstein, J.L.; Julien, J.-P. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun. 2017, 8, 764.
- Leonard, J.P.; Coleman, M.; Ketas, J.; Ashe, M.; Fiore, J.M.; Furman, R.R.; Niesvizky, R.; Shore, T.; Chadburn, A.; Horne, H.; et al. Combination Antibody Therapy With Epratuzumab and Rituximab in Relapsed or Refractory Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2005, 23, 5044–5051.
- Pierpont, T.M.; Limper, C.B.; Richards, K.L. Past, Present, and Future of Rituximab—The World’s First Oncology Monoclonal Antibody Therapy. Front. Oncol. 2018, 8, 168.
- Nagata, S.; Ise, T.; Onda, M.; Nakamura, K.; Ho, M.; Raubitschek, A.; Pastan, I.H. Cell membrane-specific epitopes on CD30: Potentially superior targets for immunotherapy. Proc. Natl. Acad. Sci. USA 2005, 102, 7946–7951.
- Horie, R.; Watanabe, T.; Morishita, Y.; Ito, K.; Ishida, T.; Kanegae, Y.; Saito, I.; Higashihara, M.; Mori, S.; Kadin, M.E.; et al. Ligand-independent signaling by overexpressed CD30 drives NF-κB activation in Hodgkin–Reed-Sternberg cells. Oncogene 2002, 21, 2493–2503.
- Guo, F.; Sun, A.; Wang, W.; He, J.; Hou, J.; Zhou, P.; Chen, Z. TRAF1 is involved in the classical NF-κB activation and CD30-induced alternative activity in Hodgkin’s lymphoma cells. Mol. Immunol. 2009, 46, 2441–2448.
- Barta, S.K.; Gong, J.Z.; Porcu, P. Brentuximab vedotin in the treatment of CD30+ PTCL. Blood 2019, 134, 2339–2345.
- Ranuhardy, D.; Suzanna, E.; Sari, R.M.; Hadisantoso, D.W.; Andalucia, R.; Abdillah, A. CD30, CD15, CD50, and PAX5 expressions as diagnostic markers for Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL). Acta Med. Indones. 2018, 50, 104–109.
- Lawrence, C.E.; Hammond, P.W.; Zalevsky, J.; Horton, H.; Chu, S.; Karki, S.; Desjarlais, J.R.; Carmichael, D.F. XmAb™2513, an Fc Engineered Humanized Anti-CD30 Monoclonal Antibody, Has Potent In Vitro and In Vivo Activities, and Has the Potential for Treating Hematologic Malignancies. Blood 2007, 110, 2340.
- Álvarez, B.; Escalona, Z.; Uenishi, H.; Toki, D.; Revilla, C.; Yuste, M.; del Moral, M.G.; Alonso, F.; Ezquerra, A.; Domínguez, J. Molecular and functional characterization of porcine Siglec-3/CD33 and analysis of its expression in blood and tissues. Dev. Comp. Immunol. 2015, 51, 238–250.
- Crocker, P.R.; McMillan, S.J.; Richards, H.E. CD33-related siglecs as potential modulators of inflammatory responses. Ann. New York Acad. Sci. 2012, 1253, 102–111.
- Läubli, H.; Alisson-Silva, F.; Stanczak, M.A.; Siddiqui, S.S.; Deng, L.; Verhagen, A.; Varki, N.; Varki, A. Lectin galactoside-binding soluble 3 binding protein (LGALS3BP) is a tumor-associated immunomodulatory ligand for CD33-related Siglecs. J. Biol. Chem. 2014, 289, 33481–33491.
- De Propris, M.S.; Raponi, S.; Diverio, D.; Milani, M.L.; Meloni, G.; Falini, B.; Foà, R.; Guarini, A. High CD33 expression levels in acute myeloid leukemia cells carrying the nucleophosmin (NPM1) mutation. Haematologica 2011, 96, 1548.
- Liu, J.; Tong, J.; Yang, H. Targeting CD33 for acute myeloid leukemia therapy. BMC Cancer 2022, 22, 24.
- Williams, B.A.; Law, A.; Hunyadkurti, J.; Desilets, S.; Leyton, J.V.; Keating, A. Antibody therapies for acute myeloid leukemia: Unconjugated, toxin-conjugated, radio-conjugated and multivalent formats. J. Clin. Med. 2019, 8, 1261.
- Larson, R.A.; Sievers, E.L.; Stadtmauer, E.A.; Löwenberg, B.; Estey, E.H.; Dombret, H.; Theobald, M.; Voliotis, D.; Bennett, J.M.; Richie, M. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005, 104, 1442–1452.
- Gao, Y.; Mehta, K. N-linked glycosylation of CD38 is required for its structure stabilization but not for membrane localization. Mol. Cell. Biochem. 2007, 295, 1–7.
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 2008, 88, 841–886.
- Hu, Y.; Liu, H.; Fang, C.; Li, C.; Xhyliu, F.; Dysert, H.; Bodo, J.; Habermehl, G.; Russell, B.E.; Li, W. Targeting of CD38 by the tumor suppressor miR-26a serves as a novel potential therapeutic agent in multiple myeloma. Cancer Res. 2020, 80, 2031–2044.
- Irimia, R.M.; Gerke, M.B.; Thakar, M.; Ren, Z.; Helmenstine, E.; Imus, P.H.; Ghiaur, G.; Leone, R.; Gocke, C.B. CD38 Is a Key Regulator of Tumor Growth By Modulating the Metabolic Signature of Malignant Plasma Cells. Blood 2021, 138, 2652.
- Sanchez, L.; Wang, Y.; Siegel, D.S.; Wang, M.L. Daratumumab: A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J. Hematol. Oncol. 2016, 9, 51.
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N. Engl. J. Med. 2016, 375, 754–766.
- Hoseini, S.S.; Cheung, N.K. Acute myeloid leukemia targets for bispecific antibodies. Blood Cancer J. 2017, 7, e522.
- Angelova, E.; Audette, C.; Kovtun, Y.; Daver, N.; Wang, S.A.; Pierce, S.; Konoplev, S.N.; Khogeer, H.; Jorgensen, J.L.; Konopleva, M.; et al. CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica 2019, 104, 749–755.
- Patnaik, M.M.; Mughal, T.I.; Brooks, C.; Lindsay, R.; Pemmaraju, N. Targeting CD123 in hematologic malignancies: Identifying suitable patients for targeted therapy. Leuk. Lymphoma 2021, 62, 2568–2586.
- Muñoz, L.; Nomdedéu, J.F.; López, O.; Carnicer, M.J.; Bellido, M.; Aventín, A.; Brunet, S.; Sierra, J. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica 2001, 86, 1261–1269.
- Aldinucci, D.; Poletto, D.; Gloghini, A.; Nanni, P.; Degan, M.; Perin, T.; Ceolin, P.; Rossi, F.M.; Gattei, V.; Carbone, A.; et al. Expression of Functional Interleukin-3 Receptors on Hodgkin and Reed-Sternberg Cells. Am. J. Pathol. 2002, 160, 585–596.
- Jin, L.; Lee, E.M.; Ramshaw, H.S.; Busfield, S.J.; Peoppl, A.G.; Wilkinson, L.; Guthridge, M.A.; Thomas, D.; Barry, E.F.; Boyd, A. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor α chain, eliminates human acute myeloid leukemic stem cells. Cell. Stem. Cell. 2009, 5, 31–42.
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018, 2, 848–858.
- Ma, H.; Padmanabhan, I.S.; Parmar, S.; Gong, Y. Targeting CLL-1 for acute myeloid leukemia therapy. J. Hematol. Oncol. 2019, 12, 41.
- Neumann, K.; Castiñeiras-Vilariño, M.; Höckendorf, U.; Hannesschläger, N.; Lemeer, S.; Kupka, D.; Meyermann, S.; Lech, M.; Anders, H.-J.; Kuster, B.; et al. Clec12a Is an Inhibitory Receptor for Uric Acid Crystals that Regulates Inflammation in Response to Cell Death. Immunity 2014, 40, 389–399.
- Bakker, A.B.H.; van den Oudenrijn, S.; Bakker, A.Q.; Feller, N.; van Meijer, M.; Bia, J.A.; Jongeneelen, M.A.C.; Visser, T.J.; Bijl, N.; Geuijen, C.A.W.; et al. C-Type Lectin-Like Molecule-1: A Novel Myeloid Cell Surface Marker Associated with Acute Myeloid Leukemia. Cancer Res. 2004, 64, 8443–8450.
- Haubner, S.; Perna, F.; Köhnke, T.; Schmidt, C.; Berman, S.; Augsberger, C.; Schnorfeil, F.M.; Krupka, C.; Lichtenegger, F.S.; Liu, X.; et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia 2019, 33, 64–74.
- Kenderian, S.S.; Ruella, M.; Shestova, O.; Klichinsky, M.; Kim, M.; Soderquist, C.; Bagg, A.; Singh, R.; Richardson, C.; Young, R. Targeting CLEC12A with chimeric antigen receptor T cells can overcome the chemotherapy refractoriness of leukemia stem cells. Biol. Blood Marrow Transplant. 2017, 23, S247–S248.
- Xiaoxian, Z.; Shweta, S.; Cecile, P.; Jingsong, Z.; Eric, D.H.; Arie, A.; Wouter, K. Targeting C-type lectin-like molecule-1 for antibody-mediated immunotherapy in acute myeloid leukemia. Haematologica 2010, 95, 71–78.
- Gilliland, D.G.; Griffin, J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood 2002, 100, 1532–1542.
- Rasko, J.E.; Metcalf, D.; Rossner, M.T.; Begley, C.G.; Nicola, N.A. The flt3/flk-2 ligand: Receptor distribution and action on murine haemopoietic cell survival and proliferation. Leukemia 1995, 9, 2058–2066.
- Rusten, L.S.; Lyman, S.D.; Veiby, O.P.; Jacobsen, S.E. The FLT3 ligand is a direct and potent stimulator of the growth of primitive and committed human CD34+ bone marrow progenitor cells in vitro. Blood 1996, 87, 1317–1325.
- Poubel, C.P.; Mansur, M.B.; Boroni, M.; Emerenciano, M. FLT3 overexpression in acute leukaemias: New insights into the search for molecular mechanisms. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2019, 1872, 80–88.
- Radich, J.P.; Kopecky, K.J.; Willman, C.L.; Weick, J.; Head, D.; Appelbaum, F.; Collins, S.J. N-ras mutations in adult de novo acute myelogenous leukemia: Prevalence and clinical significance. Blood 1990, 76, 801–807.
- Kennedy, V.E.; Smith, C.C. FLT3 Mutations in Acute Myeloid Leukemia: Key Concepts and Emerging Controversies. Front. Oncol. 2020, 10.
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312.
- Sanford, D.; Blum, W.G.; Ravandi, F.; Klisovic, R.B.; Borthakur, G.; Walker, A.R.; Garcia-Manero, G.; Marcucci, G.; Wierda, W.G.; Whitman, S.P.; et al. Efficacy and safety of an anti-FLT3 antibody (LY3012218) in patients with relapsed acute myeloid leukemia. J. Clin. Oncol. 2015, 33, 7059.
- Piloto, O.; Griesemer, M.; Nguyen, B.; Li, L.; Li, Y.; Witte, L.; Hicklin, D.J.; Small, D. The Anti-FLT3 Monoclonal Antibody EB10 Is Cytotoxic to FLT3 Inhibitor Resistant Cells In Vivo. Blood 2005, 106, 1511.
- Furusawa, Y.; Kaneko, M.K.; Kato, Y. Establishment of C20Mab-11, a novel anti-CD20 monoclonal antibody, for the detection of B cells. Oncol. Lett. 2020, 20, 1961–1967.
- Stashenko, P.; Nadler, L.M.; Hardy, R.; Schlossman, S.F. Characterization of a human B lymphocyte-specific antigen. J. Immunol. 1980, 125, 1678–1685.
- Prevodnik, V.K.; Lavrenčak, J.; Horvat, M.; Novakovič, B.J. The predictive significance of CD20 expression in B-cell lymphomas. Diagn. Pathol. 2011, 6, 33.
- Liu, Q.; Weaver, L.S.; Liewehr, D.; Venzon, D.; Stetler-Stevenson, M.; Yuan, C.M. Increased expression of CD20 and CD45 and diminished expression of CD19 are features of follicular lymphoma. Pathol. Lab. Med. Int. 2013, 5, 21.
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199.
- Bräuner-Osborne, H.; Jensen, A.A.; Sheppard, P.O.; Brodin, B.; Krogsgaard-Larsen, P.; O’Hara, P. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D1GenBank accession Nos. for GPRC5C: AF207989, for Gprc5d: AF218809 and for GPRC5D: AF209923.1. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2001, 1518, 237–248.
- Pillarisetti, K.; Edavettal, S.; Mendonça, M.; Li, Y.; Tornetta, M.; Babich, A.; Majewski, N.; Husovsky, M.; Reeves, D.; Walsh, E.; et al. A T-cell–redirecting bispecific G-protein–coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood 2020, 135, 1232–1243.
- Smith Eric, L.; Harrington, K.; Staehr, M.; Masakayan, R.; Jones, J.; Long Thomas, J.; Ng Khong, Y.; Ghoddusi, M.; Purdon Terence, J.; Wang, X.; et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019, 11, eaau7746.
- Gao, Y.; Wang, X.; Yan, H.; Zeng, J.; Ma, S.; Niu, Y.; Zhou, G.; Jiang, Y.; Chen, Y. Comparative Transcriptome Analysis of Fetal Skin Reveals Key Genes Related to Hair Follicle Morphogenesis in Cashmere Goats. PLoS ONE 2016, 11, e0151118.
- Suurs, F.V.; Lub-de Hooge, M.N.; de Vries, E.G.E.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119.
- Ma, J.; Mo, Y.; Tang, M.; Shen, J.; Qi, Y.; Zhao, W.; Huang, Y.; Xu, Y.; Qian, C. Bispecific Antibodies: From Research to Clinical Application. Front. Immunol. 2021, 12, 626616.
This entry is offline, you can click here to edit this entry!
