The number of cancers attributable to infectious agents represents over 20% of the global cancer burden. The intracellular parasite Cryptosporidium is currently considered one of the major causes of mild and severe diarrhea worldwide. However, less attention has been paid to its tumorigenic potential despite the high exposure of humans and animals to this ubiquitous parasite and the large number of epidemiological and experimental studies revealing the link between cancer and the presence of this parasite.
- Cryptosporidium,
- cancer
- Infection and cancer
- parasitology
- clorectal cancer
- colon explant
- SCID mice
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
1. Introduction
One of the biggest obstacles to increase life expectancy in the 21st century is cancer since this disease causes about 13% of human mortality [1]. Behind the strategy of treatment and early detection, it is important to work on cancer prevention, considering that between 40% and 45% of cancers are associated with preventable risk factors, including tobacco smoke, lack of physical activity, obesity, dietary factors, exposition to solar ultraviolet (UV) radiation or infectious agents [2,3]. Subsequently, reducing the burden of cancer is possible if these risk factors could be identified and if population exposure to them could be avoided or at least reduced [3]. In particular, the role of some infectious agents as carcinogens has been already recognized by the International Agency for Research on Cancer (IARC). However, additional pathogens are probably involved in specific human cancers. This review will thus be focused on the causal link between infection and cancer, including an update on the association between the infection by the protozoan Cryptosporidium and digestive cancer. Besides the several experimental and epidemiological studies that have revealed this link, mechanistic studies have shown that this parasite is able to hijack the host-cell machinery, potentially leading to a transformation of the host cell.
2. Infection, an Important Cause of Cancer
Causal associations between infectious agents and the development of human cancers have already been highlighted [4]. Overall, the total number of cancer attributable to infectious agents in 2002 was estimated at 1.9 million cases, standing for 17.8% of the global cancer burden. Moreover, it has been hypothesized that, by 2050, the majority of human cancers could be due to infections [5,6]. However, proving that infectious agents are causative factors of human cancer remains difficult for many reasons, such as:
-
The periods between primary infection and malignant transformation are frequently very long [7].
-
Even if the majority of the infectious agents associated to human cancers are ubiquitous and common in the human population, only a small proportion of infected individuals develops cancer.
-
Some infections are linked to cancer development as associated risk factors [8].
-
Infectious agents act mainly as indirect oncogenes, without persistence of their genes within the respective cancer host cells. The most common indirect infectious carcinogens are agents causing immunosuppression, such as Human Immunodeficiency Virus (HIV) leading to Kaposi’s sarcoma, or inflammation caused by the bacteria Helicobater pylori, the trematode Schistosoma hematobium and the Hepatitis C and B viruses [7].
-
The main mechanisms by which infectious agents promote cancer are not necessarily involving direct mutagenesis, but instead are due to the complex interactions between hosts and pathogens [8].
-
An infectious agent may trigger the initial events of oncogenesis while being absent in the final tumor [7].
-
Pathogens associated with cancer are directing pathogen-driven processes leading to cell transformation. However, many non-oncogenic pathogens can also regulate these processes, indicating that other factors must be involved [8].
-
In the cases of viruses, oncogenesis can occur through the persistence of the viral genome in a latent form in an infected host cell, either without replication or through integration of the viral genome into a host-cell chromosome [8].
-
Koch’s postulates for proving a causal connection between a particular infectious agent and a disease cannot be applied to many human diseases as it would be unethical to experimentally infect humans with a potentially lethal infectious agent [8].
-
Existing diagnostic tools may not be sensitive enough to link infectious agents with cancer development or testing may occur too long after the exposure [9].
Nevertheless, at least 11 biological agents have presently been recognized by the IARC as major contributors to the global number of cancers in humans (Figure 1).
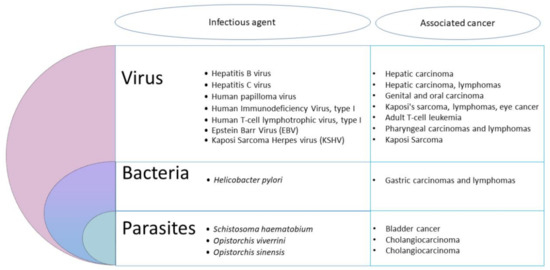
These agents include viruses, bacteria and helminths. The most important infectious agents worldwide are H. pylori (5.5% of all cancer), the human papilloma viruses (HPVs) (5.2%), the hepatitis B and C viruses (4.9%), the Epstein–Barr virus (EBV) (1%), the HIV together with the human herpes virus 8 (0.9%) and the human T-cell leukemia/lymphoma virus type 1 (HTLV-I) (0.03%) [10]. Other pathogens, including parasites, are also considered carcinogenic agents in human beings. Among helminths, the widespread digenetic trematode S. haematobium has been associated to urinary bladder cancer, and the flukes Opisthorchis viverrini and Clonorchis sinensis are causally linked with cholangiocarcinoma [10]. So, the idea of parasites as a cause of cancer in vertebrates is slowly developing [11]. However, the contribution of intracellular eukaryotic parasites to cancer development has been largely neglected until now [2]. Yet, based on clinical and epidemiological pieces of evidence, many reports underlined a potential association between parasitic protozoan infections and cancer. Hence, the flagellate Trichomonas vaginalis was suspected to be associated with prostate [12] and cervical cancers [13], while the apicomplexan Toxoplasma gondii was suggested to be linked with ocular tumor, meningioma, leukemia and lymphomas [14]. It was also suggested that Plasmodium falciparum could play a co-factor role in the development of Burkitt lymphoma [14]. Nevertheless, only the apicomplexan Cryptosporidium and Theileria have been shown to induce cell transformation experimentally [2].
Pathogens use several strategies to target cellular processes during their parasitic interactions with the host cell. The identification of microbial proteins manipulating host functions to promote infection, proliferation and escape defenses has led to great progress in understanding the host’s cellular processes. Continued persistent infection by a pathogen requires host-cell survival, host-cell proliferation, and evasion of the immune system by the pathogen. These pathogen-driven processes are achieved through various mechanisms that interfere with normal cell physiology. Alterations in these normally highly regulated pathways can lead to transforming events that have been described as the ‘hallmarks of cancer’ [5]. Carcinogenic pathogens are also able to target epigenetic mechanisms to divert the host cellular machinery [2]. Interestingly, recent mechanistic studies suggest that apicomplexan eukaryotic intracellular parasites are indeed capable of reproducing some mechanistic aspects of tumorigenesis leading to cancers, either by their infection alone, or by the combination of the parasitic infection with environmental factors [15,16]. There is a growing number of pharmacological studies analyzing the effect of anti-cancer molecules on parasitic diseases and vice versa [17]. Overall, infection seems to play a crucial role in the etiology of cancer. Actually, it was estimated that there would be 26.3% fewer cancers in developing countries and 7.7% in developed countries if cancers associated with infectious diseases were prevented [18].
3. The Special Case of Cryptosporidium: A Public Health Issue
Cryptosporidium is the agent of cryptosporidiosis, an infection resulting from the ingestion of parasite oocysts mainly through the consumption of fecally contaminated food or water, or through direct contact with the infected host [19,20]. Cryptosporidium parvum and C. hominis are the two species responsible for the majority of human cases of cryptosporidiosis. This parasite is considered a major cause of diarrhea worldwide. It causes self-limited watery diarrhea in immunocompetent individuals, but has devastating effects in those who are immunocompromised. In young children, malnutrition, growth and cognitive deficits were reported as sequels of cryptosporidiosis [21,22]. Most strikingly, a cohort study (GEMS) involving 22,500 children in Africa and Asia revealed that Cryptosporidium is one of the four main pathogens responsible for severe diarrhea and mortality in infants and toddlers [23]. This parasite was then considered the second leading cause of death in children due to diarrhea [24]. More recently, the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) analyzed in 2016 the burden of diarrhea in 195 countries and reported that Cryptosporidium is the fourth leading cause of diarrhea mortality among children under 5 years of age (with 48,301 annual death) [25,26]. It has also been reported that the substantial short-term burden of diarrhea induced by Cryptosporidium infection on childhood growth and well-being is largely underestimated [25,27] probably due to a significant proportion of asymptomatic or mild and self-limiting infections, which consequently remain not diagnosed. In addition, Cryptosporidium species are responsible for numerous waterborne outbreaks of gastrointestinal disease. The most extensive was described in 1993 in Milwaukee, USA, where over 400,000 people became ill (the population of this area was approximately 1.61 million) with 69 deaths [28,29]. The number of outbreaks caused by Cryptosporidium is increasing worldwide since 239 waterborne outbreaks were reported in Europe, Australia and North America between 2011 and 2016 versus only 120 in the same area between 2004 and 2010 [30,31]. The Centers for Disease Control and Prevention (CDC) also published that 32 outbreaks were caused by Cryptosporidium in the United States in 2016 and linked them to swimming pools and water playgrounds against only 13 in 2013 [32]. As a result, an ever-growing number of people would be exposed to this pathogen and not only in developing countries. Despite its prevalence and impact on public health, neither treatment nor vaccine against Cryptosporidium are yet available.
4. Cryptosporidium and Cancer: A Growing Body of Evidence
In various animal groups and in humans, epidemiological and experimental studies tend to reinforce the hypothesis of an association between Cryptosporidium infection and cancer (Figure 2).
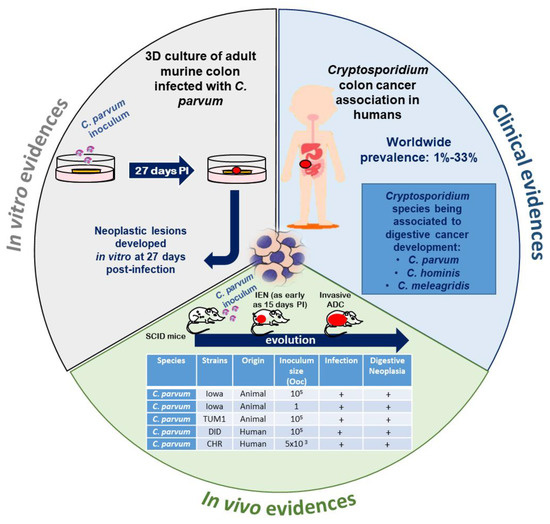
Figure 2. Recent experimental and clinical approaches contributing to expanding the understanding of the role of Cryptosporidium in the induction of digestive neoplasia. PI: post-infection, IEN: intraepithelial neoplasia, ADC: adenocarcinoma, Ooc: oocysts. Source of pictograms: https://fr.freepik.com/photos-vecteurs-libre/banner”>Banner vecteur créé par pch.vector—fr.freepik.com</a>.
5. Hypotheses about Molecules and Mechanisms Involved in the Induction of Tumorigenesis by Cryptosporidium
The pathophysiological mechanisms of Cryptosporidium infection are multifactorial and not completely understood. Some advances were achieved recently and revealed that the infection by C. parvum induces cytoskeleton remodeling and actin reorganization through the implication of several intracellular signals involving, for example, PI3K, Src, Cdc42 and GTPase [62,63] (Figure 3).
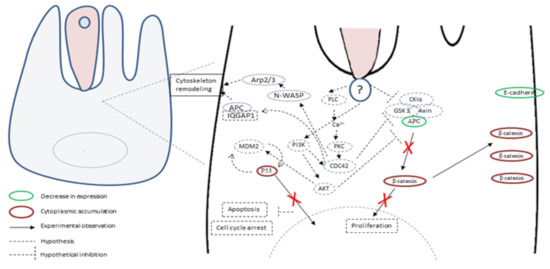
Consistently, signal transduction pathways targeting cell proliferation, cellular junctions and adhesions have also been described in gastric cancer induced by H. pylori [64]. It was also reported that the infection by C. parvum leads to the activation of NF-κB [62], known to induce anti-apoptotic mechanisms and also to transmit oncogenic signals to epithelial cells [65,66]. In addition, microarray assays were recently performed on C. parvum IId Human Ileocaecal Adenocarcinoma (HCT-8)-infected cells. A differential profile of mRNAs was found between infected and non-infected cells. Indeed, mRNAs of the Wnt and hedgehog signaling pathways were significantly differentially expressed in infected cells compared to not infected ones [67]. Noticeably, these two pathways are also involved in the development and progression of colorectal cancer [68]. Despite the growing evidence about the hijacking of cellular pathways potentially being involved in cancer onset, this information has rarely been linked to the tumorigenic potential of the parasite. To our knowledge, only one study tried to decipher this process and highlighted the important role of the Wnt signaling pathway and the alteration of the cytoskeleton in the carcinogenic process induced by C. parvum experimental infection [69]. Indeed, the immunohistochemical analysis of ileocecal region sections embedded in paraffin from C. parvum infected versus non-infected mice showed alterations in APC, β-catenin, P53 and E-cadherin expression [69]. APC and E-cadherin labelings were decreased while those of β-catenin and P53 were increased in the cytoplasm of epithelial cells. In addition, the immunofluorescence analysis of these histological sections confirmed a membranous and juxtamembraneous localization of β-catenin without nucleus translocation, suggesting an involvement of the non-canonical Wnt pathway [69]. However, unlike Helicobacter pylori, for which bacterial virulence factors associated to the gastric cancer outcome were identified, such as cytotoxin associated gene A (CagA), vacuolating cytotoxin A (VacA) and outer inflammatory protein A (OipA) [70], virulence factors implied in the C. parvum-induced carcinogenic process remain unknown. The whole genome sequences of different species and isolates of Cryptosporidium are now available [71,72,73]. Hence, a comparative genomic analysis of different C. parvum strains (Did, TUM1, CHR and Iowa, the reference strain) with variable virulence was recently performed [58]. Overall, 125 common SNVs (single nucleotide variation) (corresponding to 90 CDSs (coding sequences) were found in the three more virulent strains (Did, TUM1 and CHR) compared to Iowa strain. The majority of these genes are over-expressed in the intracellular stages of the parasite. In addition, mucins, transporters (ABC and ATPase3) and cysteine proteases were also found, these genes being already described as virulence factors. This study also reported new potential factors involved in the virulence of C. parvum such as various phosphatases (PP2A, Cdc14) and a histone-lysine N-methyltransferase. Further investigations are needed to elucidate carcinogenic mechanisms induced by C. parvum. In particular, the biological function study of the potential virulent and/or carcinogenic factors identified could be facilitated by the use of genome-editing tools such as CRISPR/Cas9 [58].
6. Conclusions and Future Directions
Is colon cancer a cause or a consequence of Cyptosporidium infection? We presented an updated picture of the link between digestive cancer and infection by this parasite. Available experimental and clinical data synthesized herein suggest that the parasite is able to employ strategies to target cellular processes during its complex interactions with host cells, leading to a parasite-induced transformation. However, the fact that Cryptosporidium is an opportunistic agent is one of the main difficulties for proving its role in the induction of human digestive cancers. In addition, available experimental models with little or no immune response are not necessarily reflecting the human condition. Despite several pieces of evidence about associations between cryptosporidiosis and digestive neoplasia, it seems that not enough attention has been paid to the tumorigenic power of this protozoan parasite. Therefore, it is necessary to demonstrate a direct causal link, and to identify virulent factors and carcinogenic mechanisms responsible for the epithelial cell transformation. A potential solution would be to combine recent advances in 3D culture models, comparative genomic studies and transfection methods (CRISPR/Cas9 system) together with further clinical trials using sensitive diagnostic tools. If the causal link between Cryptosporidium and human cancer is clearly established, a great number of digestive cancers could be prevented using public health measures to reduce the risk of Cryptosporidium infection. In addition, this could entice researchers to explore new therapeutic targets and vaccines in order to clear or prevent the infection, thereby saving hundreds of thousand children from severe diarrhoea and mortality [24]. Research into this topic is urgently needed since the incidence of Cryptosporidium infection is increasing worldwide.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms8111665
