Chronic neuropathic pain is a very important public health issue with profound negative implications in many aspects of patients’ individual lives, as well as society, health systems, productivity, and macroeconomics. The development of imaging, especially molecular and functional imaging, provides objectivity and makes the connection between structural changes, receptors involved in the mechanisms of action, and potentially therapeutic or diagnostic molecules by highlighting the place of action and the involved systems. The approval of composite biomarkers, including serological, genetic, clinical, and imaging markers, with high sensitivity and specificity will accelerate and improve diagnosis, staging, predictive and prognostic evaluation, stratification (phenotyping) and inclusion in trials, and the development of therapeutic options (pharmacological, biomedical) through preclinical, translational, clinical studies.
- neuropathic pain
- imaging biomarkers
- MRI
- PET
- MRS
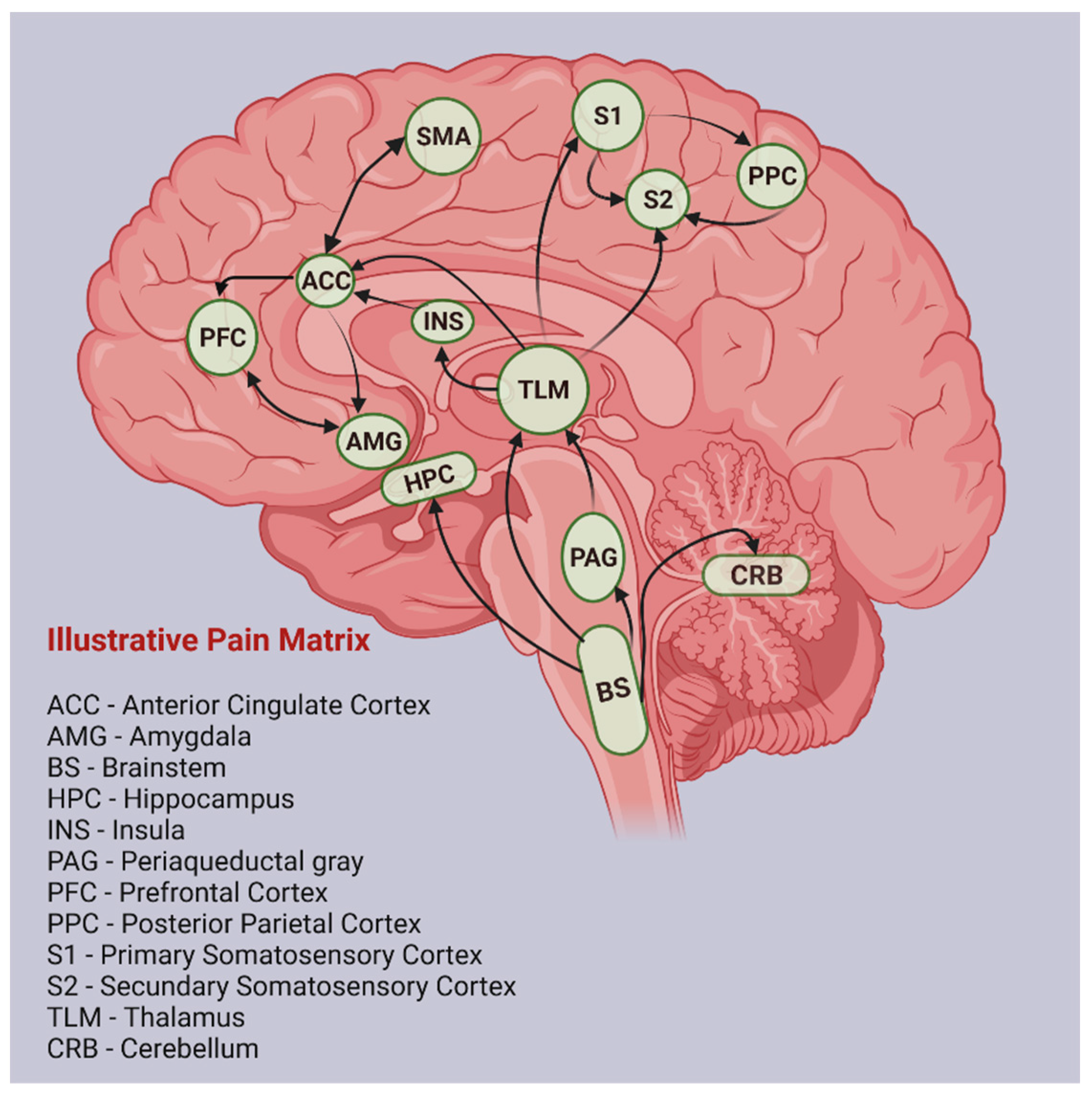
1. Chronic Neuropathic Pain and the Pain Matrix
1.1. Classification and Etiology
1.2. Structural and Functional Changes in CNP
1.3. Biochemical Changes in CNP
Understanding the long-term modifications of the peripheral and central nervous system after chronic pain has greatly increased in the past few years due to emerging preclinical research techniques, mostly performed in vitro. Such approaches are useful in understanding sensory neuron functions, based on their sensory and nociceptor-specific molecular profiles [17], i.e., ion channels and neuropeptides. The best-known ion channels involved in CNP are the voltage-activated sodium channels (Nav) [18], the voltage-gated calcium channels (Cav/VGCC), the calcium-activated potassium channels (KCa), the purinergic receptor (P2X), the transient receptor potential vanilloid family ion channel 1 (TRPV1), and the transient receptor potential cation channel ankyrin 1 (TRPA1) [19]. Of the neuropeptides, calcitonin-gene-related peptide (CGRP), substance P (SP), galanin, somatostatin and its receptors, endothelin-1 (ET1), angiotensin II and its receptors, isolectin B4 (IB4), neurotrophins, nitric oxide synthase (NOS), gamma amino butyric acid (GABA), and phospholipase β3 are known as molecules characteristic of nociceptors [20].
2. Current Imaging Techniques Used in CNP
Several imaging technologies are currently used for providing objective structural and functional measurements of different brain regions involved in the perception of pain. The best-known imaging tools are magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT), electroencephalography (EEG), and magnetoencephalography (MEG) [21].
MRI is a versatile technique that produces cross-sectional high-resolution images using a strong magnet and radio waves. From an anatomical point of view, gray matter can be assessed by MRI scanning with voxel-based morphology (VBM) or cortical thickness analysis (CTA). On the other hand, the changes caused by neuropathic pain in white matter are visible by means of MR-based diffusion tensor imaging (DTI) [22]. MRI has been used for several years in patients with chronic neuropathic pain, especially in cases that could benefit from surgery.
PET and SPECT are molecular imaging techniques with high sensitivity and good spatial resolution and penetration depth that have made a significant contribution in the evaluation of the physiological function and biochemical changes of molecular targets. Both techniques are based on the quantification of radionuclide decay, during which a positron or a γ-ray is detected. One of the main advantages that makes these imaging technologies vital, both for preclinical and clinical studies, is their capability of using highly specialized radiopharmaceutical probes tailored for specific indications, without changes in the chemical structure of the ligand [23].
EEG and MEG are electrophysiological imaging techniques that create electrical brain wave maps of various areas with high temporal resolution, but low spatial resolution and specificity. EEG collects the impulse of neural electric activity of a specific region with the aid of scalp electrodes, and MEG maps the brain activity by recording magnetic fields produced by electrical currents spontaneously occurring in the brain, using highly sensitive magnetometers [24].
3. Emerging Techniques for Diagnosing CNP
All of the imaging investigations so far offer low specificity and sensitivity for the etiologic diagnosis of neuropathic pain. It is also important to note that the diagnosis of chronic neuropathic pain is primarily based on patient symptoms, and will probably remain so in the near future. Structural and functional changes in the CNS or the peripheral nervous system do not always correlate with the presence or absence of neuropathic pain, and there is no link between the severity of the alterations and the intensity of perception. Moreover, there is still not enough data to clearly state positive and negative predictive values for any of the imaging tests discussed, most likely due to several factors: heterogeneity of clinical trial inclusion criteria, interobserver variability, technical performance of the device, different chronic pain models, and various CNP etiologies in clinical practice [10]. These important hindrances could have a significant impact on patients’ lives and lead to unnecessary exposure to expensive and potentially irradiating imaging tests [25]. As such, improving specificity and sensibility for any CNP imaging assessment considered for clinical use should be a priority, especially since some of the available imaging techniques can often link CNP to changes in the nervous system, thus aiding the diagnostic process. Of note, improvements in nerve structure or function can sometimes be an indicator that the treatment is, or will be, effective [25]. Moreover, imaging biomarkers will probably be used more and more for predicting which patients will develop CNP after a nerve lesion, and for identifying those at risk of CNP, which is particularly useful when choosing a neurotoxic treatment (as is the case with cancer patients and chemotherapy). In this sense, the use of molecular biomarkers, specific to pain-generating pathology, can identify the presence of pathological biological processes, even in the apparent absence of anatomical changes. Functional molecular and cellular imaging techniques are currently being investigated, taking advantage of heightened metabolic, hemodynamic, mediator, and cellular changes that are associated with increased nociceptive activity, in order to identify abnormal activity anywhere along the nociceptive pathways in the CNS and peripheral nervous system.
Chronic neuropathic pain is associated with a state of hyperexcitability, resulting from alteration of the excitation threshold, which leads to an increase in action potential. These changes occur due to the increase in the number and activity of voltage pumps dependent on Na and Ca, allowing increased activity of the nerves, as identified by PET studies [26]. The voltage-gated sodium channels Nav1.7 and Nav1.8 are highly expressed in sensory neurons, and have been identified as promising targets for the development of new analgesics [27]. Immunofluorescence assays and in vitro co-culture models using DRG and dorsal horn (DH) neurons in a three-compartment microfluidic platform showed that while blocking presynaptic Nav1.7 and Nav1.8 channels is effective in reducing synaptic transmission in uninjured cultures, the same blockers are ineffective in cultures where the DRG axons in the periphery compartment had been axotomized [18]. Live cell imaging and surface channel labelling in primary rat DRG neuron cultures showed that treatment with paclitaxel (PTX), which causes dose-limiting chemotherapy-induced peripheral neuropathy (CIPN), is associated with increased levels of endogenous Nav1.7 channels in DRG and long-distance axonal vesicular transport in sensory axons [28]. An immunofluorescence assay with immortalized DRG neuronal cell line (differentiated F11 cell line) identified several potential neuroprotective drugs, such as α-lipoic acid, pregabalin, and melatonin, and confirmed the same effect of felodipine and nitrendipine [29].
| Issue | Question | Preclinical Imaging Approach |
|---|---|---|
| Validation | Does the therapeutic target play a key role in the pathophysiological mechanism? | MRI whole-body scan |
| Biodistribution | Does the molecule reach the targeted tissue? Can pharmacologically active concentrations be reached for the compound being studied? | Radiolabeling of the compound to study the distribution or interactions in the target area (use of PET, SPECT, or MRS) |
| Interactions | Does the studied molecule interact with the target receptor? What is the relationship between dose and effect? | Saturation studies (plasma concentration–occupancy–time) using PET or SPECT radiolabeling |
| Pharmacodynamics | What is the effect and duration of the compound? | Dynamic biochemical studies, structural or functional studies (non-ionizing radiation imaging techniques are recommended for long-term effects follow-up) |
| Toxicology | Does the compound have acute or chronic toxic effects? | Dynamic biochemical studies, structural or functional studies of at risk organs (MRI, CT, US) |
This entry is adapted from the peer-reviewed paper 10.3390/ijms232113038
References
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.C.; Treede, R.D. A New Definition of Neuropathic Pain. Pain 2011, 152, 2204–2205.
- The Pathophysiology of Neuropathic Pain. Available online: https://www.practicalpainmanagement.com/pain/neuropathic/pathophysiology-neuropathic-pain (accessed on 1 February 2022).
- Rudin, M. Noninvasive Structural, Functional, and Molecular Imaging in Drug Development. Curr. Opin Chem. Biol. 2009, 13, 360–371.
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and Validation of Biomarkers to Aid the Development of Safe and Effective Pain Therapeutics: Challenges and Opportunities. Nat. Rev. Neurol. 2020, 16, 381–400.
- Woolf, C.J.; Mannion, R.J. Neuropathic Pain: Aetiology, Symptoms, Mechanisms, and Management. Lancet 1999, 353, 1959–1964.
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Primers 2017, 3, 17002.
- Baron, R. Mechanisms of Disease: Neuropathic Pain--a Clinical Perspective. Nat. Clin. Pract. Neurol. 2006, 2, 95–106.
- Iannetti, G.D.; Mouraux, A. From the Neuromatrix to the Pain Matrix (and Back). Exp. Brain Res. 2010, 205, 1–12.
- Salomons, T.v.; Iannetti, G.D.; Liang, M.; Wood, J.N. The “Pain Matrix” in Pain-Free Individuals. JAMA Neurol. 2016, 73, 755.
- Moisset, X.; Bouhassira, D. Brain Imaging of Neuropathic Pain. Neuroimage 2007, 37 (Suppl. 1), S80–S88.
- Tracey, I.; Mantyh, P.W. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007, 55, 377–391.
- Elbert, T.; Flor, H.; Birbaumer, N.; Knecht, S.; Hampson, S.; Larbig, W.; Taub, E. Extensive Reorganization of the Somatosensory Cortex in Adult Humans after Nervous System Injury. Neuroreport 1994, 5, 2593–2597.
- Nikolajsen, L.; Ilkjær, S.; Krøner, K.; Christensen, J.H.; Jensen, T.S. The Influence of Preamputation Pain on Postamputation Stump and Phantom Pain. Pain 1997, 72, 393–405.
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447.
- Baliki, M.N.; Geha, P.Y.; Apkarian, A.V.; Chialvo, D.R. Beyond Feeling: Chronic Pain Hurts the Brain, Disrupting the Default-Mode Network Dynamics. J. Neurosci. 2008, 28, 1398–1403.
- Scholz, J.; Woolf, C.J. The Neuropathic Pain Triad: Neurons, Immune Cells and Glia. Nat. Neurosci. 2007, 10, 1361–1368.
- Haberberger, R.V.; Barry, C.; Matusica, D. Immortalized Dorsal Root Ganglion Neuron Cell Lines. Front. Cell. Neurosci. 2020, 14, 184.
- Vysokov, N.; McMahon, S.B.; Raouf, R. The Role of Na V Channels in Synaptic Transmission after Axotomy in a Microfluidic Culture Platform. Sci. Rep. 2019, 9, 12915.
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite Glial Cells in Sensory Ganglia Express Functional Transient Receptor Potential Ankyrin 1 That Is Sensitized in Neuropathic and Inflammatory Pain. Mol. Pain 2020, 16, 1744806920925425.
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271.
- Martucci, K.T.; Mackey, S.C. Imaging Pain. Anesthesiol. Clin. 2016, 34, 255–269.
- Davis, K.D. Neuroimaging of Pain: What Does It Tell Us? Curr. Opin. Support. Palliat. Care 2011, 5, 116–121.
- Lu, F.-M.; Yuan, Z. PET/SPECT Molecular Imaging in Clinical Neuroscience: Recent Advances in the Investigation of CNS Diseases. Quant. Imaging Med. Surg. 2015, 5, 433–447.
- He, B.; Yang, L.; Wilke, C.; Yuan, H. Electrophysiological Imaging of Brain Activity and Connectivity—Challenges and Opportunities. IEEE Trans. Biomed. Eng. 2011, 58, 1918–1931.
- Diaz, M.M.; Caylor, J.; Strigo, I.; Lerman, I.; Henry, B.; Lopez, E.; Wallace, M.S.; Ellis, R.J.; Simmons, A.N.; Keltner, J.R. Toward Composite Pain Biomarkers of Neuropathic Pain-Focus on Peripheral Neuropathic Pain. Front. Pain Res. 2022, 3, 869215.
- Hoehne, A.; Behera, D.; Parsons, W.H.; James, M.L.; Shen, B.; Borgohain, P.; Bodapati, D.; Prabhakar, A.; Gambhir, S.S.; Yeomans, D.C.; et al. A 18F-Labeled Saxitoxin Derivative for in Vivo PET-MR Imaging of Voltage-Gated Sodium Channel Expression Following Nerve Injury. J. Am. Chem. Soc. 2013, 135, 18012–18015.
- Lampert, A.; Bennett, D.L.; McDermott, L.A.; Neureiter, A.; Eberhardt, E.; Winner, B.; Zenke, M. Human Sensory Neurons Derived from Pluripotent Stem Cells for Disease Modelling and Personalized Medicine. Neurobiol. Pain 2020, 8, 100055.
- Akin, E.J.; Alsaloum, M.; Higerd, G.P.; Liu, S.; Zhao, P.; Dib-Hajj, F.B.; Waxman, S.G.; Dib-Hajj, S.D. Paclitaxel Increases Axonal Localization and Vesicular Trafficking of Nav1.7. Brain 2021, 144, 1727–1737.
- Martinez, A.L.; Brea, J.; Barro, M.; Monroy, X.; Merlos, M.; Burgueño, J.; Loza, M.I. Development of a Novel in Vitro Assay to Screen for Neuroprotective Drugs against Iatrogenic Neurite Shortening. PLoS ONE 2021, 16, e0248139.
- Yang, J.-L.; Xu, B.; Li, S.S.; Zhang, W.S.; Xu, H.; Deng, X.M.; Zhang, Y.Q. Gabapentin Reduces CX3CL1 Signaling and Blocks Spinal Microglial Activation in Monoarthritic Rats. Mol. Brain 2012, 5, 18.
- Tung, K.W.; Behera, D.; Biswal, S. Neuropathic Pain Mechanisms and Imaging. Semin. Musculoskelet. Radiol. 2015, 19, 103–111.
- Behera, D.; Shen, B.; James, M.L. Radiolabeled Sigma-1 Receptor Ligand Detects Peripheral Neuroinflammation in a Neuropathic Pain Model Using PET-MRI. In Proceedings of the 2012 World Molecular Imaging Congress, Dublin, Ireland, 5–8 September 2012; World Molecular Imaging Society: Culver City, CA, USA, 2012.
- Cabañero, D.; Ramírez-López, A.; Drews, E.; Schmöle, A.; Otte, D.M.; Wawrzczak-Bargiela, A.; Huerga Encabo, H.; Kummer, S.; Ferrer-Montiel, A.; Przewlocki, R.; et al. Protective Role of Neuronal and Lymphoid Cannabinoid CB2 Receptors in Neuropathic Pain. Elife 2020, 9, e55582.
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) Signals via G-Alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428.
- Filipiuc, L.E.; Ababei, D.C.; Alexa-Stratulat, T.; Pricope, C.V.; Bild, V.; Stefanescu, R.; Stanciu, G.D.; Tamba, B.-I. Major Phytocannabinoids and Their Related Compounds: Should We Only Search for Drugs That Act on Cannabinoid Receptors? Pharmaceutics 2021, 13, 1823.
- Komorowska-Müller, J.A.; Schmöle, A.-C. CB2 Receptor in Microglia: The Guardian of Self-Control. Int. J. Mol. Sci. 2020, 22, 19.
- Tamba, B.I.; Stanciu, G.D.; Urîtu, C.M.; Rezus, E.; Stefanescu, R.; Mihai, C.T.; Luca, A.; Rusu-Zota, G.; Leon-Constantin, M.-M.; Cojocaru, E.; et al. Challenges and Opportunities in Preclinical Research of Synthetic Cannabinoids for Pain Therapy. Medicina (B Aires) 2020, 56, 24.
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 Receptors: A Therapeutic Target for the Treatment of Inflammatory and Neuropathic Pain. Br. J. Pharmacol. 2008, 153, 319–334.
- Niu, J.; Huang, D.; Zhou, R.; Yue, M.X.; Xu, T.; Yang, J.; He, L.; Tian, H.; Liu, X.H.; Zeng, J. Activation of Dorsal Horn Cannabinoid CB2 Receptor Suppresses the Expression of P2Y 12 and P2Y 13 Receptors in Neuropathic Pain Rats. J. Neuroinflamm. 2017, 14, 185.
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639.
- Franc, B.L.; Acton, P.D.; Mari, C.; Hasegawa, B.H. Small-Animal SPECT and SPECT/CT: Important Tools for Preclinical Investigation. J. Nucl. Med. 2008, 49, 1651–1663.
- Ahn, B.-C. Applications of Molecular Imaging in Drug Discovery and Development Process. Curr. Pharm. Biotechnol. 2011, 12, 459–468.
- Matthews, P.M.; Coatney, R.; Alsaid, H.; Jucker, B.; Ashworth, S.; Parker, C.; Changani, K. Technologies: Preclinical Imaging for Drug Development. Drug Discov. Today Technol. 2013, 10, e343–e350.
- Hooker, B.A.; Tobon, G.; Baker, S.J.; Zhu, C.; Hesterman, J.; Schmidt, K.; Rajagovindan, R.; Chandran, P.; Joshi, S.K.; Bannon, A.W.; et al. Gabapentin-Induced Pharmacodynamic Effects in the Spinal Nerve Ligation Model of Neuropathic Pain. Eur. J. Pain 2014, 18, 223–237.
- da Silva, J.T.; Seminowicz, D.A. Neuroimaging of Pain in Animal Models: A Review of Recent Literature. Pain Rep. 2019, 4, e732.
