Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cancer is the second leading cause of death worldwide after cardiovascular diseases. One of the most promising targeted therapies for cancer treatment is antibody therapy. It has a superior targeting ability for antigens that are expressed on cancer cells, which results in prominent antitumor activity and lower toxicity, compared with that of chemotherapeutic agents. Recent progress in recombinant DNA technology and antibody engineering has ushered in a new era of bispecific antibody (bsAb)-based immune-cell engagers (ICEs), including T- and natural-killer-cell engagers.
- bispecific antibody
- immune-cell engager
- cancer
- therapeutic target
- T-cell
- NK cell
1. Introduction
Cancer is one of the major leading causes of death worldwide. In 2020, nearly 19.3 million new cancer cases and 10.0 million cancer deaths were reported globally. More specifically, the most common cancers among new cases were breast cancer (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers [1]. The order of the mortality rate on the basis of cancer types was as follows: lung (18%), colorectal (9.4%), liver (8.3%), stomach (7.7%), and breast (6.9%) cancers [2]. By 2040, the number of new cancer cases is expected to be approximately 28.3 million, which is nearly >50% from that reported in 2020 [3]. Currently, various therapeutic regimens, such as surgical resection, chemotherapy, antibody therapy, radiotherapy, and combination therapy, have been used in clinical practice for the effective treatment of patients with cancers, depending on their health conditions and cancer status [4].
One of the most promising targeted therapies for cancer treatment is antibody therapy. It has a superior targeting ability for antigens that are expressed on cancer cells, which results in prominent antitumor activity and lower toxicity, compared with that of chemotherapeutic agents [5]. As of December 2021, 110 therapeutic antibodies, including monoclonal antibodies (mAbs), bispecific antibodies (bsAbs), and antibody–drug conjugates (ADCs), have been approved by the United States Food and Drug Administration (US FDA) and/or the European Medicines Agency (EMA). Among them, 46 antibodies are indicated for cancer treatment [6]. Generally, immunoglobulin (Ig) G-based mAb—the most widely used mAb form for antibody therapy—comprises two heavy and two light chains. The light chain has one variable (VL) and one constant (CL) domain, whereas the heavy chain has one variable (VH) and three constant (CH; CH1–CH3) domains [7,8]. Furthermore, the fragment antigen-binding (Fab) region of mAb plays a key role in cancer therapy, and specifically in modulating or blocking the signaling pathways that are involved in cancer development; on the other hand, the fragment crystallizable (Fc) region interacts with Fc receptors that are expressed on immune cells and participates in various effector functions, such as killing cancer cells via antigen-dependent T-cellular cytotoxicity (ADCC) [9,10].
BsAbs harness the specificities of two antibodies and combine them to simultaneously recognize two independent epitopes or antigens [11]. More specifically, these antibodies are designed and manufactured to contain two target-binding units in one antibody-based molecule, whereby each unit independently recognizes its unique epitope, through quadromas, chemical conjugation, or genetic recombination [12]. Compared with mAbs in cancer therapy, bsAbs have several potential benefits, such as the improvement in therapeutic efficacy, the enhancement of tumor-cell selectivity, and the reduction in tumor-cell resistance [13]. In 2009, catumaxomab—a rat–mouse hybrid bsAb for the CD3 epithelial cell adhesion molecule (EpCAM)—was first approved by the EMA for the treatment of malignant ascites in patients with EpCAM-positive cancer [14]. However, it was voluntarily withdrawn from the US market in 2013, and from the European Union (EU) market in 2017, because of commercial reasons [15]. Since the US FDA approval of blinatumomab—a CD19 × CD3 mouse bispecific T-cell engager (BiTE) antibody—for the treatment of acute lymphoblastic leukemia (ALL) in 2014, much attention has been paid to the development of bsAb-based immune-cell engagers (ICEs) that redirect immune effector cells against cancer cells and promote antitumor activities [16,17]. Furthermore, compared with adoptive immune-cell therapy, which requires an expensive and complicated manufacturing process, ICE has become a feasible therapeutic cancer-therapy approach. Currently, increasing numbers of bsAb-based ICEs are extensively evaluated in clinical trials worldwide [18,19].
2. Action Mechanism of bsAb-Based ICEs in Cancer
BsAb-based ICEs play a key role in cancer immunotherapy, and specifically in recruiting and engaging immune effector cells that are proximal to tumor-associated antigens (TAA) that are expressed on cancer cells and that allow the formation of immune synapses and a specialized cell–cell junction between the immune and cancer cells [20,21]. Ultimately, these immune synapses promote the elimination of target cancer cells [22]. These bsAb-based ICEs are currently classified into T- and natural killer (NK)-cell engagers (Figure 1).
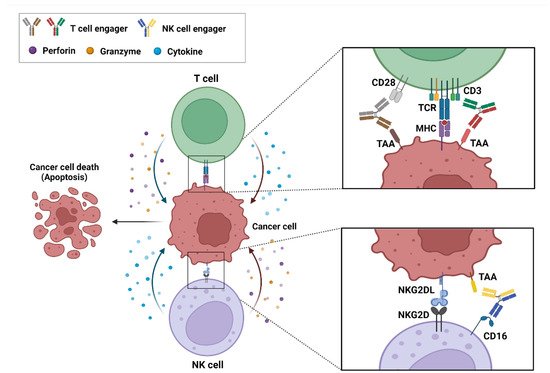
Figure 1. Action mechanism of bispecific antibody (bsAb)-based immune-cell engagers (ICEs) in cancers. The schematic drawing represents bsAb-based ICEs, including T- and natural killer-cell engagers, that bind simultaneously to tumor-associated antigens on cancer cells and specific antigens, such as CD3, CD28, and CD16 on immune cells. These interactions result in the formation of an immune synapse and the activation of immune cells that release cytokines, perforins, and granzymes to induce the cytotoxic effects on cancer cells.
3. Role of Known and Emerging Targets of ICEs
3.1. Single-Pass ICE Targets in Solid Cancers
3.1.1. Delta-Like Ligand 3
Delta-like ligand 3 (DLL3) is a 65-kDa type I transmembrane protein and a Notch receptor ligand. It plays an important role in the regulation of Notch signaling [37]. In small-cell lung cancer (SCLC), DLL3 has been reported as a key factor in the promotion of the tumor growth, migration, and invasion of SCLC cells. Several lines of evidence support this notion [37,38]. Upregulated DLL3 expression was verified to promote tumor growth in a mouse xenograft model that was implanted with DLL3-overexpressing SBC-5 human SCLC cells. Additionally, DLL3 knockdown reduces SCLC-cell migration and invasion, whereas its overexpression in the cells increases these activities [38]. This protein is highly upregulated and aberrantly expressed in SCLC and other neuroendocrine malignancies, but not in nonmalignant T-cells [37,39]. Currently, DLL3 is considered an attractive novel potential therapeutic target in neuroendocrine tumors (NETs), including SCLC. A preclinical study on robalpituzumab tesirin—an ADC that targets DLL3—showed a dose-dependent reduction in the tumor size with a complete response (CR) in B6129SF1/J mice that were implanted with DLL3-positive KP1 SCLC cells, which led to the absence of measurable tumors for >80 days after treatment [40].
3.1.2. Epidermal Growth Factor Receptor
Epidermal growth factor receptor (EGFR) is a 170-kDa receptor tyrosine kinase that belongs to the ErbB family and that comprises two major functional domains—the extracellular and cytoplasmic domains—and a tyrosine kinase domain that is linked by a single transmembrane region [41,42]. EGF binding to the receptor induces the dimerization of the receptor; triggers the autophosphorylation of cytoplasmic tyrosine residues; and eventually participates in the regulation of cell proliferation, migration, and adhesion [41,43,44,45]. EGFR is overexpressed in various cancers, such as colorectal cancer (CRC), lung cancer, breast cancer, glioblastoma, and head and neck squamous cell carcinoma. EGFR overexpression in CRC has been closely associated with tumor progression and poor prognosis [46,47,48,49,50]. Currently, EGFR is one of the most well-known therapeutic targets in various cancers. Phase II clinical studies on cetuximab—a human/mouse chimeric mAb that targets EGFR in advanced CRC—have demonstrated that the use of cetuximab as monotherapy exerts anticancer effects with approximately 10% partial response (PR) and 33% stable disease (SD) [51].
3.1.3. EpCAM
EpCAM is a 40-kDa type I transmembrane glycoprotein that plays a key role in the regulation of cell adhesion, proliferation, and differentiation [52,53,54]. EpCAM is overexpressed in various cancers, such as ovarian cancer, CRC, breast cancer, lung cancer, and pancreatic cancer [55,56,57,58]. Its protease-cleaved intracellular domain associates with β-catenin to form a nuclear protein complex that is translocated to the nucleus, activates the transcription of genes that are involved in cancer-cell proliferation, and results in tumorigenesis [52]. Several studies have suggested EpCAM as a potential target for antibody therapy against cancers. For instance, a phase II clinical study on adecatumumab (MT201)—a fully human mAb that targets EpCAM in metastatic breast cancer—reports that, of 112 patients treated with adecatumumab, 2 showed a PR and 10 had SD, according to the response evaluation criteria in solid tumors (RECIST) [59].
3.1.4. Glycoprotein A33
Glycoprotein A33 (GPA33)—also known as cell surface A33 antigen—is a 43-kDa cell surface differentiation glycoprotein that belongs to the type I transmembrane protein family [60,61,62]. It is associated with cell–cell adhesion [60]. GPA33 is highly overexpressed in >95% of human CRCs but is not detected in any other tissues [62]. Several studies have indicated GPA33 as a potential target in immunotherapy against CRC. For instance, in vivo studies on KRN330—a human mAb that targets GPA33—have demonstrated its dose-dependent antitumor activities in mouse and rat xenograft models implanted with LS174T human CRC cells [63,64].
3.1.5. Human EGFR 2
Human EGFR 2 (HER2) is a member of the ErbB family and is a 185-kDa single-pass transmembrane receptor. To the best of the researchers' knowledge, direct ligands for HER2 have not been identified yet. HER2 activation is achieved through homo- or heterodimerization with HER2 or other ErbB-family receptor members, including EGFR and HER3 [65,66,67]. It is overexpressed in various cancers, such as breast, gastric/gastroesophageal, and colon cancers [68,69,70]. HER2 is closely associated with cancer-cell proliferation and invasion, as well as with tumor growth [71,72]. Particularly in breast cancers, HER2 is overexpressed in 15–30% of the total patients with breast cancers [65]. Substantial evidence has shown that HER2 is an important predictive biomarker in HER2-targeted therapies, and a well-known therapeutic target in breast cancers. Trastuzumab is the first anti-HER2 humanized mAb that targets HER2 in breast cancer. In a phase III clinical study, patients with breast cancer treated with trastuzumab combined with chemotherapy had a longer survival (median survival, 25.1 vs. 20.3 months) and prolonged disease progression (median, 7.4 vs. 4.6 months) than those treated with chemotherapy alone [73].
3.1.6. Mucin 16
Mucin 16 (MUC16)—also known as human carbohydrate antigen 125 (CA-125)—is a heavily glycosylated 300–500-kDa type I transmembrane protein [74,75,76]. It is a biomarker for ovarian cancer. MUC16 is overexpressed in ovarian cancer and contributes to ovarian-cancer progression and metastasis [76,77]. Increased MUC16 expression is associated with poor prognosis in patients with ovarian cancer [78]. Some studies have reported that MUC16 is a potential target for antibody therapy against ovarian cancers. Oregovomab is a mouse mAb that targets MUC16 in advanced ovarian cancer. In a phase II clinical study, 145 patients with stage III/IV ovarian cancer were randomized to receive oregovomab (n = 73) or placebo (n = 72). The time to recurrence was prolonged in the oregovomab group (24.0 months) compared with that in the placebo group (10.8 months) [79].
3.1.7. Mucin 17
Mucin 17 (MUC17) is a 452-kDa type I membrane-associated mucin that is expressed on the apical surface of gastrointestinal epithelial cells [80,81,82]. As a key component of the mucosal layer, MUC17 has been suggested to play a crucial role in the restoration and protection of epithelial cells [80]. Recent studies have demonstrated that aberrant MUC17 overexpression is correlated with the malignant potential of gastric and pancreatic cancers [81,83]. Particularly in gastric-cancer tissues, MUC17 is overexpressed in approximately 50% of the gastric-cancer cases. Thus, MUC17 is a compelling target in gastric cancer because of its prevalent expression on tumor cells compared with its low, relatively restricted expression in normal tissues [84].
3.1.8. Prostate-Specific Membrane Antigen
Prostate-specific membrane antigen (PSMA) is a 100-kDa type II membrane protein, is exclusively overexpressed in prostate cancer, and acts as a glutamate-preferring carboxypeptidase. Its expression is associated with tumor invasiveness [85,86]. PSMA is not only a well-known biomarker but is also a potential therapeutic target in prostate cancer. Its expression is 100–1000-fold higher in prostate-cancer tissue than in normal tissue, and it is present on the cell surface without being released into the circulation [87,88,89,90]. Furthermore, J591 was recently developed as the first humanized mAb that targets the extracellular domain of PSMA in prostate cancer. A phase I/II study was conducted to evaluate the safety and efficacy of J591 in patients with metastatic castration-resistant prostate cancer (mCRPC). Of the 23 patients with measurable disease, 14 (60.8%) had SD and 6 (26.1%) had progressive disease, according to the RECIST [91].
3.2. Multi-Pass Transmembrane Proteins as ICE Targets in Solid Cancers
3.2.1. Claudin-18 Isoform 2
Claudin-18 isoform 2 (CLDN18.2) is a 23-kDa tetra-transmembrane protein. It plays an important role in the regulation of tight junction formation and cell adhesion [92,93,94]. It is known as a tumor-specific marker in gastric or gastroesophageal junction (GEJ) cancers because it is overexpressed exclusively in primary gastric malignancies, but not in any healthy tissues, except stomach mucosa [90,92,95]. Some studies have suggested that CLDN18.2 is a target for antibody therapy against cancer. Claudiximab (IMAB362) is a chimeric mAb that targets CLDN18.2 in gastric cancer. In a phase II study, patients with advanced/recurrent gastric and GEJ cancers who were treated with claudiximab combined with chemotherapy exhibited a significantly improved progression-free survival (PFS) (median, 7.9 vs. 4.8 months) and prolonged overall survival (OS) (median, 13.3 vs. 8.4 months) compared with those treated with chemotherapy alone [96].
3.2.2. Six-Transmembrane Epithelial Antigen of Prostate 1
Six-transmembrane epithelial antigen of prostate 1 (STEAP1) is a 39-kDa integral membrane protein that comprises six transmembrane helices [97,98]. In normal cells, STEAP1 plays a key role in the regulation of cell migration and proliferation, despite its low expression or absence in normal tissues [97,99]. It is highly overexpressed in various cancers, and particularly in prostate cancer, wherein it is involved in the regulation of various functions, such as cancer-cell invasion and proliferation, as well as tumorigenesis [98,99]. The knockdown of STEAP1 has been shown to inhibit T-cell growth in androgen-dependent prostate cancer [100]. Moreover, high STEAP1 expression is closely associated with poor outcomes in patients with prostate cancer [101]. These properties make STEAP1 a potential target for antibody therapy. DSTP3086S—an ADC that targets STEAP1—exhibited antitumor activity in a phase I clinical trial in patients with mCRPC. Of the 46 patients, 2 (4%) showed a PR and 24 (52%) had SD, according to the RECIST [102].
3.2.3. Somatostatin Receptor 2
Somatostatin receptor 2 (SSTR2) is a 41-kDa G protein-coupled receptor (GPCR), which is also known as a seven-transmembrane receptor. It is highly overexpressed in most NETs [103,104,105]. Among the NETs, and particularly in SCLC, the high expression of SSTR2 is closely associated with poor prognosis. Furthermore, the loss of SSTR2 reduced tumor growth in a mouse xenograft model implanted with H1048 human SCLC cells [104]. Several studies have suggested that SSTR2 is a therapeutic target in NETs. For instance, the antitumor efficacy of ADC that targets SSTR2 was evaluated in a mouse xenograft model implanted with BON-1 human NET cells, in which it reduced tumor growth [106].
3.3. Glycosylphosphatidylinositol-Anchored Proteins as ICE Targets in Solid Cancers
3.3.1. Carcinoembryonic Antigen
Carcinoembryonic antigen (CEA) is a 180–200-kDa member of the immunoglobulin supergene family. It plays a key role in the regulation of various cellular functions, such as cell interaction, cell adhesion, and immune response [107,108,109]. CEA is one of the most widely used tumor-marker proteins for various cancers, such as colorectal, gastric, and liver cancers [110,111,112]. It is highly overexpressed in 90% of the total CRC cases, and it is closely associated with poor prognosis in patients with CRC [113]. In CRCs, CEA is involved in cancer progression and metastasis, as well as drug resistance [110,114,115,116,117]. Furthermore, CEA appears to be a potential target for antibody therapy against CRC. A preclinical study on IMMU-130—an ADC that targets CEA—revealed that the ADC efficiently reduced tumor growth in a mouse xenograft model implanted with LS174T human CRC cells [118,119].
3.3.2. Glypican 3
Glypican 3 (GPC3) is a 60-kDa glycosylphosphatidylinositol (GPI)-anchored membrane-bound heparin sulfate proteoglycan. It plays an important role in normal cell growth [120,121]. GPC3 is overexpressed in various cancers, such as hepatocellular carcinoma (HCC), lung squamous cell carcinoma, and ovarian clear cell carcinoma [122,123,124]. In particular, it is highly overexpressed in 70–81% of HCCs; its overexpression correlates with the poor prognosis of patients with HCC [121]. Several studies have suggested that GPC3 is a target for antibody therapy against HCCs. For instance, a preclinical study on GC33—a mAb that targets GPC3—demonstrated its prominent antitumor activity in a mouse xenograft mouse model implanted with SK-HEP-1 human HCCs. The administration of 1 mg/kg of GC33 significantly inhibited tumor growth, and that of 5 mg/kg resulted in tumor remission [125].
3.4. Sphingolipid as ICE Targets in Solid Cancers
GD2
GD2 is a 1.6-kDa glycosylated lipid molecule that belongs to the class of glycosphingolipids [126,127,128,129]. It plays a key role in the attachment of tumor cells to extracellular matrix proteins. It is overexpressed in various cancers, such as neuroblastoma, melanoma, and SCLC, but not in normal tissues [127,130,131,132]. Particularly in SCLC and neuroblastoma, GD2 overexpression is involved in cell proliferation [130]. GD2 has been suggested as a target for antibody therapy against cancer. A phase II clinical study on 3F8—a mouse mAb that targets GD2 in patients with neuroblastoma—revealed that, of 16 patients, 1 showed a CR, and 1 showed a mixed response [133].
3.5. Single Transmembrane Proteins as ICE Targets in Hematological Cancers
3.5.1. B-Cell Maturation Antigen
B-cell maturation antigen (BCMA)—a member of tumor necrosis factor receptor superfamily member 17 (TNFRSF17)—is a 20-kDa type III transmembrane protein [134]. BCMA binds to its ligands, such as proliferation-inducing ligand and B-cell-activating factor, which thus promotes the survival of B-cells [135,136]. It is overexpressed in malignant plasma cells, including multiple myeloma (MM) cells, and it plays a crucial role in the growth of MM [137,138]. A preclinical study that was conducted that used a mouse xenograft model implanted with RPMI 8226 human MM cells has shown that BCMA overexpression promotes tumor growth [137]. Furthermore, high BCMA expression is associated with poor prognosis in patients with MM [139]. Substantial evidence has shown that BCMA is a target for antibody therapy against MM. Preclinical studies on belantamab mafodotin—a US FDA-approved ADC that targets BCMA—showed that it efficiently inhibited tumor growth and prolonged survival in a mouse xenograft model implanted with H929 human MM cells [140,141].
3.5.2. CD19
CD19 is a 95-kDa type I transmembrane protein [142]. It is a coreceptor of B-cell antigen receptor (BCR), and it plays a role in regulating B-cell growth [143,144]. It is overexpressed in most B-cell malignancies, such as ALL, non-Hodgkin lymphoma (NHL), and chronic lymphocytic leukemia (CLL). The overexpression of CD19 promotes the proliferation and survival of these B-cell malignancies [145,146]. Previous studies have suggested that CD19 is an attractive target for antibody therapy against B-cell malignancies. In a phase IIa clinical study on XmAb5574 (MOR00208)—a humanized mAb that targets CD19—of the total patients with relapsed and/or refractory (R/R) NHL who received XmAb5574 monotherapy, 8% showed a CR [147].
3.5.3. CD22
CD22 is a 140-kDa type I transmembrane protein and an inhibitory coreceptor of the BCR that regulates the overstimulation of B-cells [148,149]. CD22 is overexpressed in various B-cell lymphomas, such as CLL, ALL, and NHL, but it is expressed at low levels on immature B-cells and plasma cells [150,151]. Owing to the restricted expression on the B-cell and the inhibitory function of CD22, CD22 has been indicated as a therapeutic target in B-cell lymphoma [152]. Epratuzumab—a humanized mAb that targets CD22—has been reported to be a CD22 agonistic antibody that leads to B-cell inhibition [153]. In a phase II clinical trial, patients with R/R indolent or aggressive NHL were enrolled to receive epratuzumab combined with rituximab, which is a US FDA-approved anti-CD20 mAb. Of the 16 patients with indolent NHL, 9 showed a CR and an unconfirmed CR, and 1 showed a PR. Furthermore, of the six patients with aggressive NHL, three showed a CR, and one showed a PR [154,155].
3.5.4. CD30
CD30 is a 120-kDa type I transmembrane protein that belongs to the tumor necrosis factor receptor family [156]. It plays a key role in lymphocyte activation and proliferation through the nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase pathways that have antiapoptotic and prosurvival benefits [157,158]. It is overexpressed in hematopoietic malignancies, including Hodgkin lymphoma (HL) and NHL, and is associated with the survival of these cells [159,160]. Several studies have suggested that CD30 is a target for antibody therapy against hematologic malignancies. For instance, in vivo studies on XmAb2513—a humanized mAb that targets CD30—showed a significant reduction in the tumor growth, and enhanced survival was observed in a mouse xenograft model implanted with CD30-expressing L540 human HL cells [161].
3.5.5. CD33
CD33—also known as the sialic acid-binding Ig-like lectin 3 (Siglec-3)—is a 67-kDa type I transmembrane protein [162]. It plays a crucial role in the modulation of immune-cell functions, such as phagocytosis, cytokine release, and apoptosis [163,164]. It is overexpressed in acute myeloid lymphoma (AML), and its overexpression is observed in >80% of patients with AML [165]. This increased CD33 expression is correlated with the poor prognosis of patients with AML. In patients with AML who were treated with chemotherapy, the OS rate has been reported to be 42.9% in patients with high CD33 expression, compared with 67.5% in those with low CD33 expression [166]. CD33 is a target for antibody therapy against AML. Gemtuzumab ozogamicin (Mylotarg)—a US FDA-approved ADC that targets CD33—showed promising clinical efficacy in patients with AML [167]. In a phase II clinical study, patients with AML in the first recurrence received gemtuzumab ozogamicin monotherapy; of the 277 patients, 35 showed a CR, and 36 showed a CR with incomplete platelet recovery [168].
3.5.6. CD38
CD38 is a 45-kDa type II transmembrane protein [169]. It is overexpressed in MM cells but shows a low expression in normal lymphoid and myeloid cells [170]. It participates in MM cell survival and proliferation [171]. Previous studies have elucidated that tumor growth decreased in a mouse xenograft model implanted with CD38-knockout RPMI 8226 human MM cells, compared with those nontargeting cells [172]. CD38 is a potential target for antibody therapy against MM. Daratumumab (Darzalex) is the first US FDA-approved human mAb that targets CD38 in the treatment of patients with R/R MM [173]. In a phase III clinical study, patients with R/R MM received chemotherapy (control group) or chemotherapy combined with daratumumab (daratumumab group); the CR rate was significantly higher in the daratumumab group (19.2%) than in the control group (9.0%) [174].
3.5.7. CD123
CD123—the alpha chain of the interleukin-3 (IL-3) receptor—is a 75-kDa type I transmembrane protein [175]. It is overexpressed in leukemic stem cells but shows low or no expression in normal hematopoietic stem cells [176]. CD123 binds to IL-3, which is a hematopoietic growth factor, which leads to the survival and proliferation of various hematologic cancers, such as AML, ALL, and HL [175,177,178,179]. Particularly in AML, increased CD123 expression is associated with a poor prognosis of patients with AML [180]. Previous studies have suggested that CD123 is a target for antibody therapy against AML. In vivo studies on IMGN632—an ADC that targets CD123—have revealed its antitumor activities in a mouse xenograft model implanted with MOLM-13 human AML cells; the mice received IMGN632 or control ADC, and IMGN632 increased the survival of mice compared with the vehicle treatment [181].
3.5.8. C-Type Lectin Domain Family 12 Member A
C-type lectin domain family 12 member A (CLEC12A)—a myeloid inhibitory receptor—is a 31-kDa type II transmembrane protein. It plays a crucial role in the negative regulation of inflammation [182,183]. CLEC12A is specifically expressed in AML and is observed in approximately 90% of patients with AML, but not in normal hematopoietic stem and progenitor cells [184,185]. It is closely associated with the poor prognosis of patients with AML. Previous studies have shown that CLEC12A-positive AML cells are more resistant to chemotherapy than CLEC12A-negative AML cells [186]. Furthermore, the administration of anti-CLEC12A chimeric mAb showed a significant tumor-growth delay of up to 38% in a mouse xenograft model implanted with HL-60 human AML cells [187]. These observations suggest that CLEC12A is a target for antibody therapy against AML.
3.5.9. FMS-Like Tyrosine Kinase 3
FMS-like tyrosine kinase 3 (FLT3)—a receptor tyrosine kinase—is a140–160-kDa type I transmembrane protein [188]. FLT3 promotes the proliferation and differentiation of hematopoietic cells by binding to its ligand [189,190]. FLT3 is overexpressed in AML, and its mutations have been detected in approximately 30% of patients with AML [191,192]. The mutation of FLT3 causes ligand-independent FLT3 signaling and leads to a poor prognosis of patients with AML [193,194]. FLT3 is a potential target for antibody therapy against AML. LY3012218 (IMC-EB10) is a human mAb that targets FLT3, which prevents FLT3 signaling [195]. In preclinical studies, LY3012218 has shown efficacy in a mouse xenograft model implanted with MOLM-14 human AML cells; LY3012218 exerts its effects by reducing the engraftment of leukemic cells and extending survival [196].
3.6. Multi-Pass Transmembrane Proteins as ICE Targets in Hematological Cancers
3.6.1. CD20
CD20 is a 33–37-kDa tetra-transmembrane protein [197]. It is involved in B-cell differentiation and it is overexpressed in most B-cell malignancies, such as follicular lymphoma (FL), but not in hematopoietic stem cells or plasma cells [198,199,200]. Several studies have shown that CD20 is a potential target for antibody therapy against FL. Rituximab (Rituxan) is a chimeric mAb that has been approved by the US FDA against CD20 [155]. In a phase III clinical study, patients with R/R FL received lenalidomide plus rituximab or placebo plus rituximab; the median PFS increased in the lenalidomide-plus-rituximab group (39.4 months), compared with that in the placebo-plus-rituximab group (14.1 months) [201].
3.6.2. GPCR Class C Group 5 Member D
GPCR class C group 5 member D (GPRC5D) is a 39-kDa seven-transmembrane protein [202]. It is an orphan receptor that is normally expressed only in the hair follicle. GPRC5D is overexpressed in MM and is unlikely to be shed from the membrane, which prevents the decrease in the efficacy of GPRC5D-targeted therapy [203,204,205]. The role of GPRC5D in cancers is yet to be defined; nonetheless, selective GPRC5D expression may be valuable as a target for antibody therapy against MM. Figure 2 summarizes the known and emerging targets for ICE therapy against cancers.
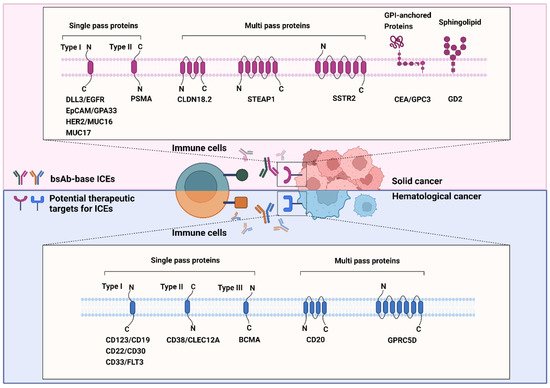
Figure 2. Known and emerging targets for immune-cell engager (ICE) therapy against cancers. The schematic representation shows known and emerging therapeutic targets of bispecific antibody-based ICEs in solid (red background) and hematological (blue background) cancers. All therapeutic targets listed in this figure are grouped on the basis of their relationship with the bilayer and transmembrane topology.
4. Design and Structure of bsAb-Based ICEs
BsAb-based ICEs are designed to contain two different antigen-binding sites that comprise determinants from the VH and VL chains of different antibodies that are specific to each target [206]. Thus far, various efforts have been made to increase their homogeneity, yield, and functional properties to generate desired bsAb-based ICEs [11]. On the basis of the bsAb-based ICE structures, they are divided into two categories: Fv-based ICEs and immunoglobulin G (IgG)-based ICEs (Figure 3). Fv-based ICEs are easy to produce and show lower immunogenicity, whereas IgG-based ICEs have higher solubility, stability, affinity, and extended half-life in serum [207].
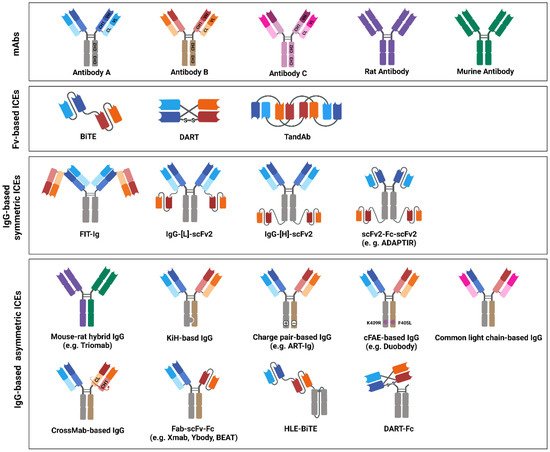
Figure 3. Immune-cell engager (ICE) structures in clinical studies, or ICEs approved by the United States Food and Drug Administration (US FDA) and/or European Medicines Agency (EMA). The schematic drawing depicts the structures of bispecific antibody (bsAb)-based ICEs that are currently evaluated in clinical studies or that have been approved by the US FDA and/or EMA. The structures of bsAb-based ICEs are subdivided into three major classes: variable fragments -based, immunoglobulin G (IgG)-based symmetric, and IgG-based asymmetric ICEs. Variable heavy-chain domains (VHs) of two different antibodies, designated as antibodies A, B, or C, are shown in dark blue, red, or pink, respectively. The variable light-chain domains (VLs) are shown in light blue, red, and pink, respectively. Moreover, rat or mouse antibody is depicted in purple or green, respectively.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23105686
This entry is offline, you can click here to edit this entry!
