Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
Mitochondria are highly dynamic organelles with the ability to continuously cleavage and fuse, regulating dynamic homeostatic processes in response to the needs of organism growth and the changes in external environmental conditions.
- mitochondria
- morphological and dynamic changes
- mitochondrial ultrastructure
1. Introduction
Plant mitochondria are two membranous organelles of endosymbiotic origin with their own genetic information. As the energy factory of cells, the main functions of plant mitochondria are producing ATP through tricarboxylic acid cycle and oxidative phosphorylation, releasing energy and producing various metabolic products to participate in programmed cell death (PCD), oxidative stress, and other key cellular processes. All of them are crucial for the maintenance of homeostasis in eukaryotes and the growth of organisms.
In higher plants, mitochondria are usually spherical, sausage-shaped, linear, or network shaped, and their morphology is highly variable [1]. They are usually distributed in the cytoplasm [2] with diameters ranging from 0.2 to 1.5 μm [3]. Mitochondria dynamic processes can be divided into fusion and fission. They can fuse and connect with each other to form network-like structures or split to form dispersed individuals. The movement of mitochondria depends on cytoskeleton, which is composed of actin filaments, intermediate filaments, and microtubules [4]. They move rapidly along tubulin and actin filaments in cellular mitochondria of which processes are regulated by protein kinases [5,6]. Based on their morphological and dynamic characteristics, mitochondria can adjust their shape, number, and orientation according to the developmental needs of plant cells [7,8]. Such morphological and dynamic changes will eventually lead to changes in mitochondrial functions, which gives them the ability to adapt to changes in the external environment.
With the global warming, plants on land are constantly affected by various adverse or even negative environmental conditions. Among them, abiotic stress (e.g., high temperature, low temperature, salt, drought, ozone, UV radiation, etc.) has adverse effects on plant growth and productivity [9]. Plants respond to abiotic stress in various ways. One of the most obvious features is the induction of excessive cellular production of reactive oxygen species (ROS). Ultimately, it may lead to PCD [10].
In plant cells, the molecular and physiological responses of mitochondria to stress are well known. As a powerhouse organelle in eukaryotes, mitochondria produce large amounts of ATP, which can provide 95% of the energy required for life activities. It is the main site for regulating apoptosis and producing ROS [11]. Therefore, plant mitochondria play a key role in the process of responding to abiotic stress. Under stress, plant mitochondria sense metabolic changes, such as pH, energy status, and ROS, and respond to them by inducing permeability transition pores (PTP) and releasing cytochrome c. When stress persists and intracellular ROS exceed the regulatory threshold of mitochondria, they will break down and lose functions, triggering a cascade of PCD in cells [12,13,14,15,16], which ultimately leads to the obstruction of plant growth and development. Experiments have confirmed that mitochondria produce a large number of ROS and the loss of outer membrane potential under various stimuli, which were considered to be the early product of PCD in Arabidopsis thaliana [17].
2. Morphological and Dynamic Changes in Mitochondria
2.1. Ultrastructure of Mitochondria
Mitochondria are two membrane-closed organelles whose morphological characteristics include four functional regions from the outside to the inside: outer membrane (OM), intermembrane space (IMS), inner membrane (IM), and mitochondrial matrix (Figure 1). Mitochondrial outer membrane separates the mitochondria from the cytoplasmic matrix. Their IM can be divided according to its different functions into inner boundary membrane (IBM), which is close to and parallel to OM and cristae membrane (CM), which bulges toward the matrix [18,19]. Notably, CM is not a simple extension and folding of IM. Instead, it is connected to IBM through a narrow tubular cavity called cristae junctions (CJs) [19,20], which have been described as the third compartment of mitochondria [21]. IBM and CM have different topologies and protein compositions, where IBM is located next to OM and possesses the mechanism by which most proteins enter inner mitochondria. While CM is the main site of oxidative phosphorylation and contains the complex of respiratory chain as well as F1Fo-ATP synthase [22,23]. An increasing number of studies has demonstrated that the shape of CJs is related to the process of mitochondrial fusion and fission [24,25], especially to PCD [22,26,27].
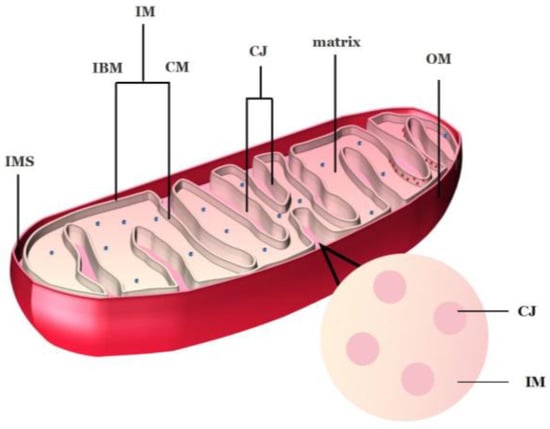
Figure 1. Mitochondrial structure of plants. IMS: intermembrane space; IM: inner membrane; CM: cristae membrane; CJ: cristae junctions; IBM: inner boundary membrane; OM: outer membrane. The blue spots in the matrix are ribosomes; the red spots in the CM are mitochondrial respiratory chain complexes. Image credit: Hui Tang.
2.2. Structure of Cristae
In plants, although significant progress has been made in identifying proteins involved in mitochondrial morphology, little is known about the protein complexes that control CJs biogenesis. In yeast and mammals, the large GTPase optic atrophy 1 (OPA1), the mitochondrial contact site and cristae organizing system (MICOS), and the IM protein dimer F1Fo-ATP synthase are involved in the regulation of cristae morphology [22,27,28]. Mammalian OPA1 (Mgm1 in yeast) is involved in remodeling and fusion of the mitochondrial inner membrane [29,30,31]. In addition, MICOS is an evolutionarily conserved large heterooligomeric protein complex of IM, mainly located in CJs [32,33,34,35]. MICOS is composed of two different subcomplexes, with MIC10 and MIC60 (also known as mitogen) as the core components [18], which maintain the stability of normal mitochondrial endometrium cristae and are crucial for the formation of cristae [33,36,37]. The overexpression of Mic60 and Mic10 leads to the bifurcation of cristae [38] and the strong expansion and deformation of CM and CJs [39]. In plants, according to phylogenetic analysis of eukaryotes, only MIC60 and MIC10 are conserved [40,41].
MICOS, as a large multi-subunit complex involved in mitochondrial biogenesis and stability, is closely related to the maintenance of mitochondrial structure. The absence of several MICOS subunits leads to intense changes in mitochondrial cristae morphology, including the loss of cristae and the formation of cristae stack (which appear as stacks within the mitochondrial matrix) [19,42,43]. Moreover, lipid exchange is also necessary to maintain the integrity of the mitochondrial membrane. An Arabidopsis study has shown that MIC60 interacts with the mitochondrial outer membrane transportase (TOM) through the TOM 40-kD subunit (TOM40) to form part of the mitochondrial transmembrane lipoprotein (MTL) complex, which influences the mitochondrial lipid transport [44]. In addition, Mic60, together with the mitochondrial outer membrane protein DGD1 suppressor 1 (DGS1), forms multi-subunit complexes in Arabidopsis [45]. The loss of DGS1 resulted in the whole plant physiology being affected, namely it altered the stability and protease accessibility of this complex and altered the lipid content and composition of the mitochondria, particularly causing changes in the abundance and size of cristae in the morphology [45].
The energy conversion of the mitochondrial respiratory chain is achieved by electrochemical proton gradient inside and outside the mitochondrial inner membrane. This potential gradient is then utilized by the F1F0-ATP synthase to produce ATP. The F1Fo-ATP synthase, one of the mitochondrial electron transport chains (ETC) complexes, i.e., complex V, exists as a dimer and is found along the most tightly curved regions of the cristae ridge or around narrow tubular fractures [22,46,47]. In plants, ATP synthase accounts for about 8.44% of the volume of the mitochondrial inner membrane, which is assembled together with complex I~IV on mitochondrial cristae to form multiple and bulky ETC complexes, which jointly affect the mitochondrial inner membrane structure [48]. It has been proved that the dimer state of F1Fo-ATP synthase can affect the structure of cristae [48] and the deletion of ATP synthase subunits e and g leads to “onion-like” structures of cristae [28,47,49]. Strauss and Hofhaus [50] have found that the control of ATP synthase on cristae morphology and the ATP synthase would exert a strong local curvature on the membrane, resulting in the formation of cristae so that protons would gather in the cristae. At this time, mitochondrial cristae act as a proton trap, and ATP synthase can achieve more efficient ATP synthesis by changing the mitochondrial inner membrane morphology driven by strong proton power [50].
Mitochondrial membrane structure and cristae shape are also affected by some proteins that affect membrane stability. Membrane-anchored ATP-dependent metalloproteinases called FtsH4 or AAA proteases are believed to be key enzymes for the quality control of membrane proteins in mitochondria and chloroplasts [51]. The absence of AtFtsH4 changed the leaf morphology of the rosette at the later stage of development, and the number of mitochondrial cristae decreased significantly in the micromorphology of organelles during the short-day cycle [51].
At present, the molecular mechanism of the morphological change in plant mitochondrial cristae is not perfect, as a result of the structure of plant cells is more complex. In order to fill this gap, maybe we can refer to the above reports to promote the study of plant mitochondrial morphology.
2.3. Dynamic Changes in Mitochondria in Plants
2.3.1. Proteins Associated with Mitochondrial Fusion
The dynamic processes of mitochondria depend on the proteins involved in the regulation of mitochondrial formation, fusion, and fission.
In mammals and yeast, the dynamin-related protein 1 DLP1/Drp1(the yeast homologous Dnm1p), which is a superfamily member of large guanosine triphosphatases (GTPases), mediates the dynamic process of mitochondrial division [52]. Located mainly in the cytoplasm, DLP1/Drp1 has a highly conserved NH2-terminal GTPase domain at the N-terminal, followed by an intermediate domain, and a GTPase effect-domain (GED) with mitochondrial targeting at the C-terminal [53]. Mitochondrial fission requires recruitment of Drp1 to the mitochondrial surface and activation of its GTP-dependent fission function. In mammals, the primary receptors that recruit Drp1 to facilitate mitochondrial fission include mitochondrial fission 1 protein (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51, respectively). They drive DRP1-mediated division by interacting [54] or recruiting different subgroups of Drp1 [55]. There are mitochondrial fission factors in Arabidopsis, dynamin-related proteins DRP3A and DRP2B (ADL2a and ADL2b, the functional orthologs of Dnm1p and DLP1/Drp1), thought to be key factors in both mitochondrial and peroxisomal fission. In the double mutant, drp3a/drp3b, the mitochondria are connected to each other, resulting in massive elongation [56]. They are functionally redundant in mitochondrial fission, but the frequency of mitochondrial fission in Arabidopsis depends on the total abundance of DRP3A and DRP3B [56]. In Arabidopsis thaliana, there are two closely related Fis1 homologues (i.e., FIS1A and FIS1B) that have been reported to target mitochondria, peroxisome, and chloroplast, which have been shown to promote mitochondrial fission [57,58]. From now on, there is no other homologue of the mitochondrial fission factor (Mdv1p/Caf4p, Mff or MiD49/51) in Arabidopsis thaliana. However, there are Arabidopsis-specific fission factors--elongated mitochondria 1 (ELM1) and peroxisome and mitochondrial division factors (PMDs). ELM1 localizes to the outer surface of mitochondria and mediates mitochondrial fission by recruiting DRP3A; moreover, mitochondria in elm1 mutant showed a slender shape [59]. However, elm1 mutant has residual mitochondrial fission activity [59], which may be caused by a completely independent mitochondrial fission system. It has been reported that elongated mitochondria had been observed in pmd1 mutants; however, PMD1 did not physically interact with DRP3 or FIS1 [60]. It suggests that PMD1 may promote mitochondrial proliferation in an independent manner from DRP3/FIS1.
2.3.2. Proteins Associated with Mitochondrial Fission
Mitochondrial fusion is also regulated by large GTPases, which requires three steps: the connection of two mitochondria, fusion of the OM, and fusion of IM [61,62].
Mitofusin (Mfn)1 and Mfn2, (homolog Fzo1 in yeast) are involved in mitochondrial tethering and OM fusion. In the absence of Mfn1 or Mfn2 cells, the imbalance of the fusion and fission events leads to mitochondrial fragmentation, leading to severe mitochondrial and cellular dysfunction [63,64]. Mfns structurally contain the C-terminal GTPases region, middle region (MD), transmembrane region (TM, including TM1 and TM2), and the GTPase effector domain (GED). MD and GED are two predicted heptad repeat domains (HR1 and HR2). The sequence composed of eleven amino acids in the middle of TM1 and TM2 region was identified as mitochondrial targeting sequence MTS. It facilitates anchoring and targeting of these proteins to the mitochondrial membrane [65]. The hetero-type complex formed by Mfn1-Mfn2 or the single homologous Mfn1 or Mfn2 complex are important regulators of mitochondrial outer membrane attachment and fusion [64,66].
Human optic atrophy-1 (OPA1) (homologue Mgm1 in yeast) is mitochondrial localization protein, which is a member of the dynamin family. Thus, it has the characteristic structure of the dynamin family, including N-terminal mitochondrial targeting signals (MTS) and transmembrane domains, GTPase domain, a central domain, and a GED at the C-terminal. When the OPA1 imports into mitochondria, its matrix-targeting signal is removed, and as an L-isoform tightly bound to or embedded in IM, the rest resides in the mitochondrial intermembrane space [67,68]. In general, OPA1 mediates IM fusion and maintains the cristae structure. When OPA1 is deactivated, it can cause mitochondria to fragment [69].
From now on, no specific fusion factor has been found in plants. Mitochondria often presents as highly fragmented and suggests that plants with the dynamical process of mitochondria are mainly composed of fission. However, fusion phenomenon does occur. For example: mitochondria in lower plants, such as the unicellular freshwater alga Micrasterias denticulata, are globular and exist independently of each other in the cytosol at room temperature. As the temperature decreases, mitochondria begin to aggregate and fuse with each other. When subjected to extracellular freezing stress at −2 °C, Micrasterias mitochondria are aggregated into local networks, and their OMs are connected or fused with each other and finally aggregated into a large mitochondrial network [70]. In higher plants, Arimura [71] demonstrated the fusion of mitochondria through an interesting phenomenon. They labeled mitochondria in onion epidermal cells with mitochondria-targeted, photoconvertible fluorescent protein Kaede and then used light processing to turn some of the mitochondria within a cell from green to red. Finally, they found the appearance of yellow mitochondria.
2.3.3. Fusion and Fission of Cardiolipin with Mitochondria
The composition of the mitochondrial membrane is also critical to the dynamic process of mitochondrial fusion and fission. Mitochondrial membrane is composed of phosphatidic acid (PA), phosphatidylserine (PS), cardiolipin (CL), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylcholine (PC), and phosphatidylinositol (PI), in which PC and PE are the main components. One of the potential mechanisms by which lipids affect mitochondrial morphology may be due to their ability to recruit and/or activate proteins that mediate the process of fission and fusion. For example, as a precursor for glycerol lipid synthesis, which is produced by Mt-GPAT functions, LPA produced on OM may stimulate GTPase activity of Mfns and enhance mitochondrial fusion during OM fusion [72]. Similar stories were reported in CL. CL is unique in mitochondria and significantly enriched in IM, accounting for 10–20% of total phospholipids [73], and even up to 25% at the contact site between IM and OM [74]. In yeast, CL binds to the soluble form of Mgm1 in order to stimulate its GTPase activity and affect the MGM1-mediated IM fusion process [75]. Additionally, its cardiolipin unsaturation level is the key to mitochondrial functions and IM integrity [76]. In the mitochondria of Arabidopsis thaliana, cardiolipin content in ftsh4 mutant leads to the deregulation of mitochondrial dynamics and causes perturbations within the OXPHOS complexes, which generates more reactive oxygen species and less ATP [77]. In addition, CL is also involved in the remodeling of the highly folded cristae of mitochondria [78] and affects mitochondria-mediated apoptosis and other mitochondrial functional processes. It is reported that CL is a key determinant for mt-DNA stability and segregation during mitochondrial stress [79]. Thus, these results further demonstrate the importance of CL not only for mitochondrial functions but also for the health of the organism as a whole.
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae9010011
This entry is offline, you can click here to edit this entry!
