Fucales are an order within the Phaeophyceae that include most of the common littoral seaweeds in temperate and subtropical coastal regions. Many species of this order have long been a part of human culture with applications as food, feedand remedies in folk medicine. Apart from their high nutritional value, these seaweeds are also a well-known reservoir of multiple bioactive compounds with great industrial interest. Among them, phlorotannins, a unique and diverse class of brown algae-exclusive phenolics, have gathered much attention during the last few years due to their numerous potential health benefits. However, due to their complex structural features, combined with the scarcity of standards, it poses a great challenge to the identification and characterization of these compounds, at least with the technology currently available. Nevertheless, much effort has been taken towards the elucidation of the structural features of phlorotannins, which have resulted in relevant insights into the chemistry of these compounds.
- Phaeophyceae
- brown algae
- sargassaceae
- fucaceae
- phlorotannins
- phenolic compounds
- structural elucidation
- mass spectrometry
- NMR
1. Introduction
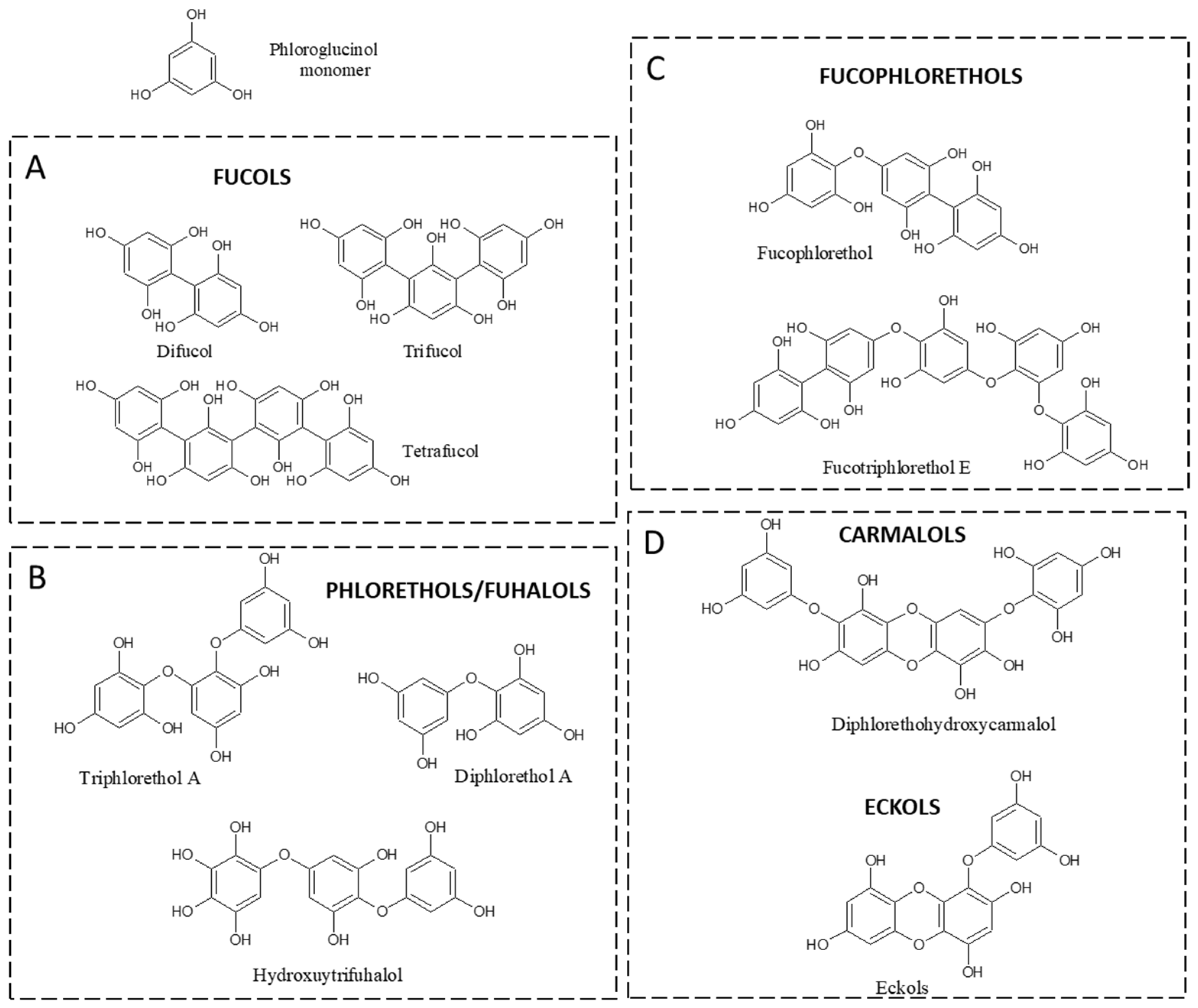
2. Phlorotannins and Main Classes in Fucale
3. Extraction Processes
This entry is adapted from the peer-reviewed paper 10.3390/md20120754
References
- Cho, G.Y.; Rousseau, F.; de Reviers, B.; Boo, S.M.; Reviers, B.D.E.; Cho, G.Y.; Rousseau, F.; Reviers, B.D.E. Phylogenetic Relationships within the Fucales (Phaeophyceae) Assessed by the Photosystem I Coding PsaA Sequences. Phycologia 2006, 45, 512–519.
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106.
- Bermejo, R.; Chefaoui, R.M.; Engelen, A.H.; Buonomo, R.; Neiva, J.; Ferreira-Costa, J.; Pearson, G.A.; Marbà, N.; Duarte, C.M.; Airoldi, L.; et al. Marine Forests of the Mediterranean-Atlantic Cystoseira tamariscifolia Complex Show a Southern Iberian Genetic Hotspot and No Reproductive Isolation in Parapatry. Sci. Rep. 2018, 8, 10427.
- Montero, L.; Herrero, M.; Ibáñez, E.; Ibá, I.; Ibáñez, I.; Cifuentes, A. Separation and Characterization of Phlorotannins from Brown Algae Cystoseira abies-marina by Comprehensive Two-Dimensional Liquid Chromatography. Electrophoresis 2014, 35, 1644–1651.
- Jégou, C.; Connan, S.; Bihannic, I.; Cérantola, S.; Guérard, F.; Stiger-Pouvreau, V. Phlorotannin and Pigment Content of Native Canopy-Forming Sargassaceae Species Living in Intertidal Rockpools in Brittany (France): Any Relationship with Their Vertical Distribution and Phenology? Mar. Drugs 2021, 19, 504.
- Guiry, M.D.; Guiry, G.M.; Sargassum, C. Agardh, 1820—AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=77 (accessed on 4 November 2022).
- Amador-Castro, F.; García-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of Pelagic Sargassum Biomass into Sustainable Applications: Current Trends and Challenges. J. Environ. Manag. 2021, 283, 112013.
- Daniel, S.L.; Kiril, B.; Leonel, P. Production of Bio-Fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Oceanol. Limnol. 2019, 37, 918–927.
- Ghaffar Shahriari, A.; Mohkami, A.; Niazi, A.; Hamed Ghodoum Parizipour, M.; Habibi-Pirkoohi, M. Application of Brown Algae (Sargassum angustifolium) Extract for Improvement of Drought Tolerance in Canola (Brassica napus L.). Iran. J. Biotechnol. 2021, 19, e2775.
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Optimization of Biogas Production from Sargassum Sp. Using a Design of Experiments to Assess the Co-Digestion with Glycerol and Waste Frying Oil. Bioresour. Technol. 2015, 175, 480–485.
- Giovanna Lopresto, C.; Paletta, R.; Filippelli, P.; Galluccio, L.; de la Rosa, C.; Amaro, E.; Jáuregui-Haza, U.; Atilio de Frias, J. Sargassum Invasion in the Caribbean: An Opportunity for Coastal Communities to Produce Bioenergy Based on Biorefinery—An Overview. Waste Biomass Valorization 2022, 13, 2769–2793.
- Luis Godínez-Ortega, J.; Cuatlán-Cortés, J.V.; López-Bautista, J.M.; van Tussenbroek, B.I. A Natural History of Floating Sargassum Species (Sargasso) from Mexico. In Natural History and Ecology of Mexico and Central America; IntechOpen: London, UK, 2021.
- Soleimani, S.; Yousefzadi, M.; Nezhad, S.B.M.; Pozharitskaya, O.N.; Shikov, A.N. Evaluation of Fractions Extracted from Polycladia Myrica: Biological Activities, UVR Protective Effect, and Stability of Cream Formulation Based on It. J. Appl. Phycol. 2022, 34, 1763–1777.
- Serrão, E.A.; Alice, L.A.; Brawley, S.H. Evolution of the Fucaceae (Phaeophyceae) Infrred from NrDNA-ITS. J. Phycol. 1999, 35, 382–394.
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208.
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling Phlorotannins from Fucus Spp. of the Northern Portuguese Coastline: Chemical Approach by HPLC-DAD-ESI/MSn and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120.
- Stansbury, J.; Saunders, P.; Winston, D. Promoting Healthy Thyroid Function with Iodine, Bladderwrack, Guggul and Iris. J. Restor. Med. 2013, 1, 83–90.
- Guiry, M.D.; Guiry, G.M. Fucus Linnaeus, 1753—AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org/search/genus/detail/?genus_id=71 (accessed on 4 November 2022).
- Rasul, F.; Gupta, S.; Olas, J.J.; Gechev, T.; Sujeeth, N.; Mueller-Roeber, B. Priming with a Seaweed Extract Strongly Improves Drought Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1469.
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655.
- Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.-F.; Jacques, H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs 2022, 20, 174.
- Fraser, C.I.; Vel, M.; Nelson, W.A.; Macaya, E.C.; Hay, C.H.; Mccarthy, C.; Velásquez, M.; Nelson, W.A.; Macaya, E.C.; Hay, C.H. The Biogeographic Importance of Buoyancy in Macroalgae: A Case Study of the Southern Bull-Kelp Genus Durvillaea (Phaeophyceae), Including Descriptions of Two New Species. J. Phycol. 2007, 56, 23–36.
- Capon, R.J.; Barrow, R.A.; Rochfort, S.; Jobliig, M.; Skene, C.; Lacey, E.; Gill, J.H.; Friedel, T.; Wadsworth, D.; Jobling, M.; et al. Marine Nematocides: Tetrahydrofurans from a Southern Australian Brown Alga, Notheia Anomaliz. Tetrahedron 1998, 54, 2227–2242.
- Mueller, R.; Wright, J.T.; Bolch, C.J.S.S. Historical Demography and Colonization Pathways of the Widespread Intertidal Seaweed Hormosira banksii (Phaeophyceae) in Southeastern Australia. J. Phycol. 2018, 54, 56–65.
- Clayton, M.N. Circumscription and Phylogenetic Relationships of the Southern Hemisphere Family Seirococcaceae (Phaeophyceae). Bot. Mar. 1994, 37, 213–220.
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–Bioactivity and Extraction Perspectives. J. Appl. Phycol. 2022, 34, 2173–2185.
- Hermund, D.B.; Torsteinsen, H.; Vega, J.; Figueroa, F.L.; Jacobsen, C. Screening for New Cosmeceuticals from Brown Algae Fucus vesiculosus with Antioxidant and Photo-Protecting Properties. Marine Drugs 2022, 20, 687.
- Lashika Blue Filter Sunscreen SPF 45 PA+++ with Brown Seaweed—30 mL. Available online: https://www.lashika.in/products/blue-filter (accessed on 16 November 2022).
- Hello Sunny Essence Sun Stick Glow SPF50+ Pa++++. Available online: https://incidecoder.com/products/banila-co-hello-sunny-essence-sun-stick-glow-spf50-pa (accessed on 16 November 2022).
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of Soluble, Cell-Wall-Bound and Exuded Phlorotannins in the Brown Alga Fucus vesiculosus, with Implications on Their Ecological Functions. J. Chem. Ecol. 2005, 31, 195–212.
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules 2015, 20, 1118–1133.
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681.
- Kim, S.M.; Kang, S.W.; Jeon, J.-S.; Jung, Y.-J.; Kim, W.-R.; Kim, C.Y.; Um, B.-H. Determination of Major Phlorotannins in Eisenia bicyclis Using Hydrophilic Interaction Chromatography: Seasonal Variation and Extraction Characteristics. Food Chem. 2013, 138, 2399–2406.
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and Temporal Variation in Phlorotannin Levels in an Assemblage of Brown Algae. Bot. Mar. 2004, 47, 410–416.
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can Phlorotannins Purified Extracts Constitute a Novel Pharmacological Alternative for Microbial Infections with Associated Inflammatory Conditions? PLoS ONE 2012, 7, e31145.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The Biochemical Composition and Antioxidant Properties of Fucus vesiculosus from the Arctic Region. Mar. Drugs 2022, 20, 193.
- Pedersen, A. Studies on Phenol Content and Heavy Metal Uptake in Fucoids. In Eleventh International Seaweed Symposium. Developments in Hydrobiology; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; Volume 22, pp. 498–504.
- Connan, S.; Stengel, D.B. Impacts of Ambient Salinity and Copper on Brown Algae: 2. Interactive Effects on Phenolic Pool and Assessment of Metal Binding Capacity of Phlorotannin. Aquat. Toxicol. 2011, 104, 1–13.
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal Variation of Phlorotannin in Sargassacean Species from the Coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61.
- Ragan, M.A.; Jensen, A. Quantitative Studies on Brown Algal Phenols. II. Seasonal Variation in Polyphenol Content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258.
- Pavia, H.; Toth, G.B. Influence of Light and Nitrogen on the Phlorotannin Content of the Brown Seaweeds Ascophyllum nodosum and Fucus vesiculosus. Hydrobiologia 2000, 440, 299–305.
- Pavia, H.; Brock, E. Extrinsic Factors Influencing Phlorotannin Production in the Brown Alga Ascophyllum nodosum. Mar. Ecol. Prog. Ser. 2000, 193, 285–294.
- Tala, F.; Velásquez, M.; Mansilla, A.; Macaya, E.C.; Thiel, M. Latitudinal and Seasonal Effects on Short-Term Acclimation of Floating Kelp Species from the South-East Pacific. J. Exp. Mar. Biol. Ecol. 2016, 483, 31–41.
- Sardari, R.R.R.R.; Prothmann, J.; Gregersen, O.; Turner, C.; Karlsson, E.N. Identification of Phlorotannins in the Brown Algae, Saccharina Latissima and Ascophyllum nodosum by Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Tandem Mass Spectrometry. Molecules 2021, 26, 43.
- Tierney, M.S.; Soler-Vila, A.; Rai, D.K.; Croft, A.K.; Brunton, N.P.; Smyth, T.J. UPLC-MS Profiling of Low Molecular Weight Phlorotannin Polymers in Ascophyllum nodosum, Pelvetia canaliculata and Fucus spiralis. Metabolomics 2014, 10, 524–535.
- Catarino, M.D.; Silva, A.A.M.S.; Cruz, M.T.; Mateus, N.; Silva, A.A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897.
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin Extracts from Fucales Characterized by HPLC-DAD-ESI-MSn: Approaches to Hyaluronidase Inhibitory Capacity and Antioxidant Properties. Mar. Drugs 2012, 10, 2766–2781.
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162.
- Li, Y.; Fu, X.; Duan, D.; Liu, X.; Xu, J.J.J.; Gao, X. Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 2017, 15, 49.
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant Capacities of Phlorotannins Extracted from the Brown Algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883.
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247.
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675.
- Michalak, I.; Chojnacka, K. Algal Extracts: Technology and Advances. Eng. Life Sci. 2014, 14, 581–591.
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B.; Mayer, A.M. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230.
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A Review of Extraction Methods, Structural Characteristics, Bioactivities, Bioavailability, and Future Trends. Algal Res. 2021, 60, 102484.
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to Control Hyperglycemia and Diabetes-Related Vascular Complications. J. Appl. Phycol. 2019, 31, 3143–3152.
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198.
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-Assisted Extraction of Bioactive Compounds from Brown Seaweeds and Characterization. J. Appl. Phycol. 2020, 32, 615–629.
- Ank, G.; Antônio Perez Da Gama, B.; Pereira, R.C. Latitudinal Variation in Phlorotannin Contents from Southwestern Atlantic Brown Seaweeds. PeerJ 2019, 7, e7379.
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Seasonal Variation of Chemical Composition and Biomethane Production from the Brown Seaweed Ascophyllum nodosum. Bioresour. Technol. 2016, 216, 219–226.
- Hermund, D.B.; Heung, S.Y.; Thomsen, B.R.; Akoh, C.C.; Jacobsen, C. Improving Oxidative Stability of Skin-Care Emulsions with Antioxidant Extracts from Brown Alga Fucus vesiculosus. J. Am. Oil Chem. Soc. 2018, 95, 1509–1520.
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250.
- Sumampouw, G.A.; Jacobsen, C.; Getachew, A.T. Optimization of Phenolic Antioxidants Extraction from Fucus vesiculosus by Pressurized Liquid Extraction. J. Appl. Phycol. 2021, 33, 1195–1207.
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave Assisted Extraction of Phenolic Compounds from Four Economic Brown Macroalgae Species and Evaluation of Their Antioxidant Activities and Inhibitory Effects on α-Amylase, α-Glucosidase, Pancreatic Lipase and Tyrosinase. Food Res. Int. 2018, 113, 288–297.
- Čagalj, M.; Skroza, D.; Tabanelli, G.; Özogul, F.; Šimat, V. Maximizing the Antioxidant Capacity of Padina pavonica by Choosing the Right Drying and Extraction Methods. Processes 2021, 9, 587.
- Amarante, S.J.; Catarino, M.D.; Marçal, C.; Silva, A.M.S.; Ferreira, R.; Cardoso, S.M. Microwave-Assisted Extraction of Phlorotannins from Fucus vesiculosus. Mar. Drugs 2020, 18, 559.
