Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The Suzuki coupling is a transition metal-catalyzed, cross-coupling carbon–carbon (C–C) bond forming reaction between organic boron compounds and organic halides. As an operationally simple and versatilely applicable procedure, the Suzuki coupling reaction has found immense applications in drug discovery and development in the pharmaceutical industry.
- Suzuki reaction
- transition metal catalyst
- catalyst recycling
1. Introduction
The Suzuki coupling reaction, also known as the Suzuki-Miyaura cross-coupling reaction, is one of the most versatile reactions to form carbon–carbon (C–C) bonds and involves a cross-coupling of organoboranes with an aryl halide in the presence of a transition metal catalyst, a ligand, and an aqueous base. The air- and moisture-stabilities, flexibility of substrates, and excellent reaction yields have led this reaction into the eminent position in synthetic organic chemistry.
The Suzuki coupling reaction has been extensively utilized in the synthesis of various industrially important compounds, such as olefins, styrenes, and substituted biphenyls [1]. For instance, Frederick et al. recently reported a total synthesis of abemaciclib using the Suzuki coupling reaction [2]. Abemaciclib is a compound that blocks the growth of malignant cells by inhibiting cell cycle progression and hence, has a consequential application as a bioactive anti-cancer drug in the pharmaceutical industry [3][4]. The Suzuki coupling reaction successfully introduced a C–C bond between boronic ester and pyrimidine to form a building block of abemaciclib. The Suzuki coupling reaction played a pivotal role for the synthesis of many other drugs and late-stage drug candidates, such as rucaparib, merestinib, and lapatinib [5].
While the Suzuki coupling reaction has been an irreplaceable part of pharmaceutical synthesis, the process heavily relies on harsh reaction conditions, toxic reagents, and copious amounts of solvents, which could cause health- and environment-related concerns and problems [6][7]. To address these issues, the concept of green chemistry was introduced to the industry in the 1990s [8]. Green chemistry is the design of chemical products or processes that maximize the product yield while reducing the use or generation of hazardous substances and hence, navigate the industry toward sustainable manufacturing. From the business point of view, green chemistry has also brought manufacturers considerable advantages, such as reduction in production cost, faster manufacturing, capacity increase, and energy savings, ultimately leading to more profitable outcomes [8]. To date, a myriad of studies has been conducted to develop sustainable synthetic processes by renovating the feedstocks, reaction conditions, and purification and isolation methods for the Suzuki coupling reaction [8][9][10].
One propitious approach for sustainable synthesis is to develop a recyclable transition metal catalyst that can be completely removed from the reaction mixture and is reusable in consecutive runs without a significant loss of catalytic performance. In the conventional Suzuki coupling process, the costly metal catalyst is not only discarded as it loses activity during the first run but may also be incorporated into the final product as a contaminant since complete removal is hardly achievable. Therefore, it has been evident that the development of a novel recyclable catalytic system is of great importance to minimize health- and environment-related concerns as well as the production cost.
2. Principles of the Suzuki Coupling Reaction
The Suzuki coupling reaction is a versatile method to form a C–C bond between an organoboronic nucleophile and an organic electrophile under basic conditions in the presence of a metal catalyst and ligand [11]. As illustrated in Figure 1, the Suzuki coupling reaction begins with the formation of a catalyst–ligand complex. The organic electrophile is then introduced into the complex via oxidative addition, followed by transmetalation during which the organoboronic nucleophile is introduced into the complex. Lastly, the palladium–ligand complex is separated from the coupling product via reductive elimination.
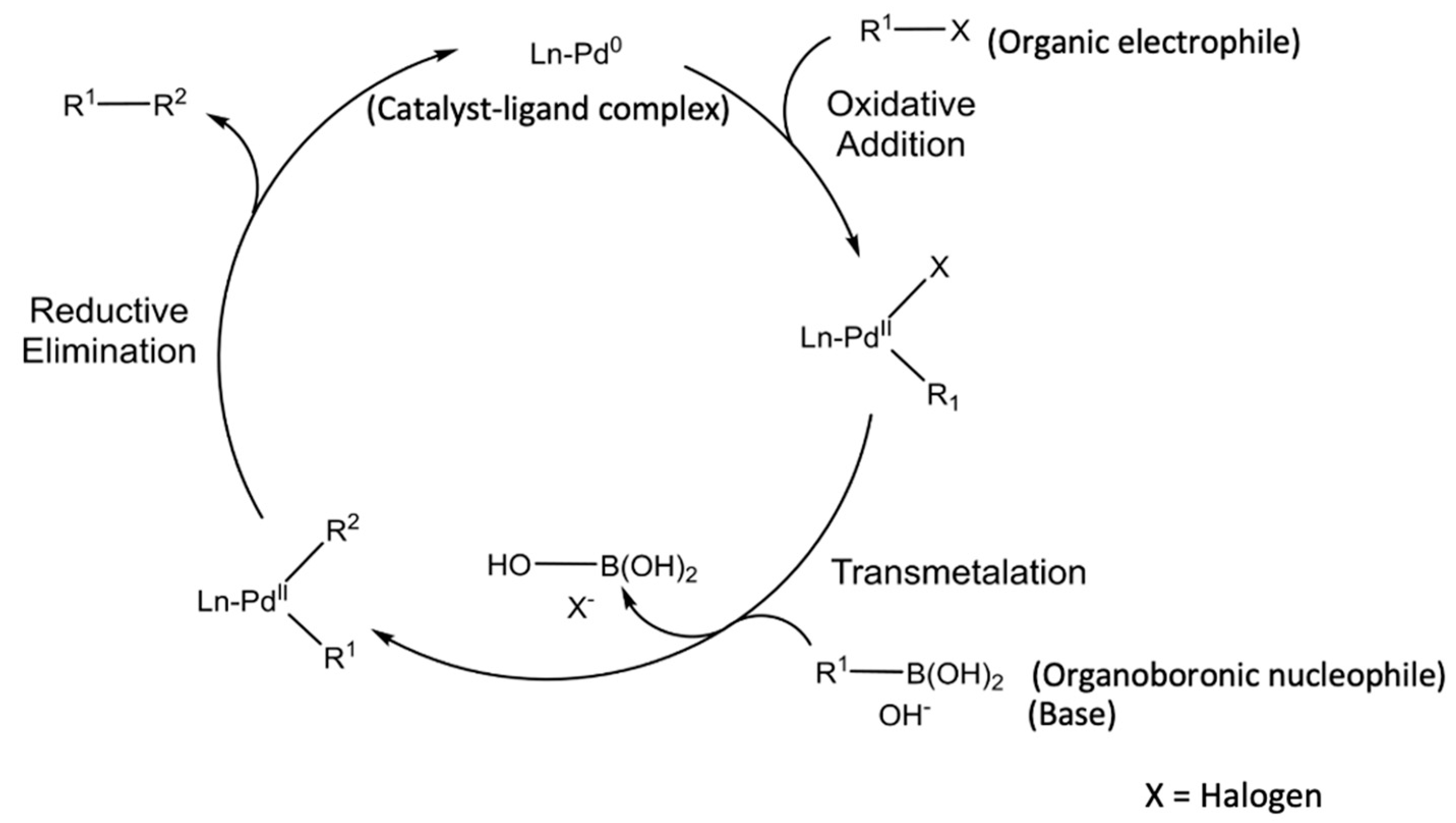
Figure 1. Catalytic cycle of the Suzuki coupling reaction [12].
A typical Suzuki coupling reaction is illustrated in Figure 2. The metal catalyst undergoes a two-electron transfer and thus, changes its oxidation state. For instance, the commonly used palladium catalyst is oxidized from Pd(0) to Pd(II) during oxidative addition to receive the organic electrophile, while Pd(II) is reduced back to Pd(0) during reductive elimination of a coupling product. The alternative Pd(II)/Pd(IV) mechanism, where Pd (IV) forms from Pd(II), has also been proposed in recent literature [13]. For either pathway, the aqueous base is essential in the reaction to convert the boronic acid to a more reactive organoborate that can be efficiently coupled with the organic electrophile [14]. The ligand joins the catalytic cycle by forming a complex with the metal catalyst and combines with the organic electrophile [15].
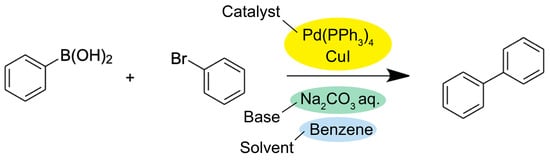
Figure 2. General reaction scheme for the Suzuki coupling reaction [16].
There are various other cross-coupling reactions that enable organic transformations via similar mechanisms, such as the Stille coupling and the Negishi coupling (Figure 3 and Figure 4). However, the Stille coupling reaction relies on organotin reagents, which are mostly toxic and expensive and have a low tolerance to functional groups [17]. As for the Negishi coupling, it suffers from low yields and low tolerance to functional groups because zinc is normally strongly bonded to carbon atoms and is less reactive toward halides [18][19]. On the other hand, the Suzuki coupling reaction offers various advantages over other coupling reactions. For instance, organoboron compounds are readily available on the market, less toxic, and highly stable to heat, oxygen, and water. In addition, a wide range of reagents can react under mild conditions. Moreover, by-products can be easily isolated from the product by extraction and/or chromatography [20][21][22]. Thus, the Suzuki coupling reaction has been widely used in the synthesis of a variety of fine chemicals and pharmaceuticals in industrial research over several decades [23][24].
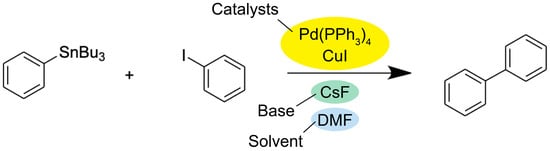
Figure 3. General reaction scheme for the Stille coupling reaction [25].
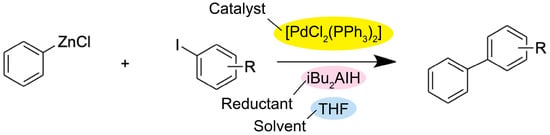
Figure 4. General reaction scheme for the Negishi coupling, where iBu2AIH is diisobutylaluminium hydride [23].
Before Suzuki and Miyaura discovered the palladium-catalyzed cross-coupling reaction of organoboronic acids, the cross-coupling reactions were limited to the use of magnesium-, manganese-, iron-, and nickel-based Grignard reagents as nucleophiles [26][27]. In 1976, Negishi demonstrated the efficacy of organonickel reagents as nucleophiles, while Heck observed the coupling reaction of boronic acid with an alkene in the presence of stoichiometric quantities of palladium [23]. It was in 1979, when Suzuki and Miyaura integrated Heck’s and Negishi’s discoveries and reported the well-known palladium-catalyzed cross-coupling reaction between 1-alkenylboranes and aryl halides, using a palladium(0) catalyst. Since then, an enormous number of studies, including the use of nickel and copper catalysts instead of palladium catalysts, have been undertaken on the Suzuki coupling reaction to synthesize various industrially important substances.
Most of these cross-coupling reactions have long relied on the use of palladium compounds as catalysts due to the superior catalytic performance and the high stability in various reaction conditions [28]. More recently, however, other transition metal catalysts, such as copper and nickel catalysts, have also been explored to develop more environmentally benign synthetic processes [29].
This entry is adapted from the peer-reviewed paper 10.3390/knowledge3010001
References
- Gujral, S.; Khatri, S.; Riyal, P. Suzuki Cross Coupling Reaction-A Review. Indo Glob. J. Pharm. Sci. 2012, 2, 351–367.
- Frederick, M.O.; Kjell, D.P. A Synthesis of Abemaciclib Utilizing a Leuckart–Wallach Reaction. Tetrahedron Lett. 2015, 56, 949–951.
- Eggersmann, T.K.; Degenhardt, T.; Gluz, O.; Wuerstlein, R.; Harbeck, N. CDK4/6 Inhibitors Expand the Therapeutic Options in Breast Cancer: Palbociclib, Ribociclib and Abemaciclib. BioDrugs 2019, 33, 125–135.
- Alison, P.; Gary, L.; Megan, S. Abemaciclib: The Newest CDK4/6 Inhibitor for the Treatment of Breast Cancer. Ann. Pharmacother. 2019, 53, 178–185.
- Schäfer, P.; Palacin, T.; Sidera, M.; Fletcher, S. Asymmetric Suzuki-Miyaura Coupling of Heterocycles via Rhodium-Catalysed Allylic Arylation of Racemates. Nat. Commun. 2017, 8, 15762.
- Gogoi, P.; Bezboruah, P.; Boruah, R.C. Ligand-Free Suzuki Cross-Coupling Reactions: Application to Β-Halo-α,Β-Unsaturated Aldehydes. Eur. J. Org. Chem. 2013, 2013, 5032–5035.
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18.
- Ratti, R. Industrial Applications of Green Chemistry: Status, Challenges and Prospects. SN Appl. Sci. 2020, 2, 263.
- Li, C.J.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202.
- Costa, N.E.; Pelotte, A.L.; Simard, J.M.; Syvinski, C.A.; Deveau, A.M. Discovering Green, Aqueous Suzuki Coupling Reactions: Synthesis of Ethyl (4-Phenylphenyl)Acetate, a Biaryl with Anti-Arthritic Potential. J. Chem. Educ. 2012, 89, 1064–1067.
- Buskes, M.J.; Blanco, M.-J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493.
- D’Alterio, M.C.; Casals-Cruañas, È.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. A Eur. J. 2021, 27, 13481–13493.
- Aghahosseini, H.; Saadati, M.R.; Rezaei, S.J.T.; Ramazani, A.; Asadi, N.; Yahiro, H.; Mori, M.; Shajari, N.; Kazemizadeh, A.R. A Robust Polyfunctional Pd(II)-Based Magnetic Amphiphilic Nanocatalyst for the Suzuki–Miyaura Coupling Reaction. Sci. Rep. 2021, 11, 10239.
- Lima, C.F.; Rodrigues, A.S.; Silva, V.L.; Silva, A.M.; Santos, L.M. Role of the Base and Control of Selectivity in the Suzuki-Miyaura Cross-Coupling Reaction. ChemCatChem 2014, 6, 1291–1302.
- Ridgway, B.H.; Woerpel, K.A. Transmetalation of Alkylboranes to Palladium in the Suzuki Coupling Reaction Proceeds with Retention of Stereochemistry. J. Org. Chem. 1998, 63, 458–460.
- Suzuki, A. Organoborane Coupling Reactions (Suzuki Coupling). Proc. Jpn. Acad. Ser. B 2004, 80, 359–371.
- Maleczka, R.E.; Gallagher, W.P.; Terstiege, I. Stille Couplings Catalytic in Tin: Beyond Proof-of-Principle. J. Am. Chem. Soc. 2000, 122, 384–385.
- Miyaura, N.; Yanagi, T.; Suzuki, A. The Palladium-Catalyzed Cross-Coupling Reaction of Phenylboronic Acid with Haloarenes in the Presence of Bases. Synth. Commun. 1981, 11, 513–519.
- García-Melchor, M.; Solans-Monfort, X.; Ujaque, G. CC Bond Formation. In Comprehensive Inorganic Chemistry II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 9, pp. 767–805.
- Maluenda, I.; Navarro, O. Recent Developments in the Suzuki-Miyaura Reaction: 2010–2014. Molecules 2015, 20, 7528–7557.
- Rau, H.H.; Werner, N.S. Stereocontrolled Synthesis of (E)-Stilbene Derivatives by Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Bioorganic Med. Chem. Lett. 2018, 28, 2693–2696.
- Martin, R.; Buchwald, S.L. Palladium-Catalyzed Suzuki−Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473.
- Seechurn, C.; Kitching, M.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085.
- Liu, J.; Lavigne, J.J. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-63932-8.
- Lee, V. Application of Copper (i) Salt and Fluoride Promoted Stille Coupling Reactions in the Synthesis of Bioactive Molecules. Org. Biomol. Chem. 2019, 17, 9095–9123.
- Seechurn, C.; De Angelis, A.; Colacot, T. Introduction to New Trends in Cross-Coupling. In New Trends in Cross-Coupling: Theory and Applications; RSC Publishing: London, UK, 2015; pp. 1–19.
- Campeau, L.-C.; Hazari, N. Cross-Coupling and Related Reactions: Connecting Past Success to the Development of New Reactions for the Future. Organometallics 2019, 38, 3–35.
- Heravi, M.M.; Hashemi, E. Recent Applications of the Suzuki Reaction in Total Synthesis. Tetrahedron 2012, 68, 9145–9178.
- Key, R.J.; Tengco, J.M.M.; Smith, M.D.; Vannucci, A.K. A Molecular/Heterogeneous Nickel Catalyst for Suzuki–Miyaura Coupling. Organometallics 2019, 38, 2007–2014.
This entry is offline, you can click here to edit this entry!
