Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is a critical agent responsible for the pandemic coronavirus disease 2019 (COVID-19). The most harmful clinical feature of COVID-19 patients is the upper
airway infection leading to severe pneumonia associated with acute respiratory distress syndrome (ARDS). The critical functional receptor for SARS-CoV-2 infection is angiotensin-converting enzyme 2
(ACE2), which belongs to the renin–angiotensin system (RAS) in humans. ACE2 is part of the non-classical RAS axis that counteract the harmful actions of the classical RAS axis. The balance of the classical ad non-classical RAS could be altered in COVID-19 patients.
- renin-angiotensin system
- COVID-19
- SARS-CoV2
- ACE2
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has produced significant health emergencies worldwide, resulting in the declaration by the World Health Organization of the coronavirus disease 2019 (COVID-19) pandemic. Acute respiratory syndrome seems to be the most common manifestation of COVID-19. A high proportion of patients require intensive care unit admission and mechanical ventilation (MV) to survive. It has been well established that angiotensin-converting enzyme type 2 (ACE2) is the primary cellular receptor for SARS-CoV-2. ACE2 belongs to the renin–angiotensin system (RAS), composed of several peptides, such as angiotensin II (Ang II) and angiotensin (1-7) (Ang-(1-7)). Both peptides regulate muscle mass and function. It has been described that SARS-CoV-2 infection, by direct and indirect mechanisms, affects a broad range of organ systems. In the skeletal muscle, through unbalanced RAS activity, SARS-CoV-2 could induce severe consequences such as loss of muscle mass, strength, and physical function, which will delay and interfere with the recovery process of patients with COVID-19.
1. Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has been responsible for significant health emergencies worldwide since the end of 2019 and throughout 2020, leading to the coronavirus disease 2019 (COVID-19) pandemic. The World Health Organization reports 13,824,739 confirmed COVID-19 cases and 591,666 deaths worldwide until July 2020 [1]. This emergency makes it urgent to identify the mechanisms of action of the virus and the possible consequences. Finding the best therapeutic strategies to treat patients with SARS-CoV-2 as soon as possible, especially those in critical condition, is an essential step to prevent more deaths and complications for those who managed to survive.
The clinical characteristics of COVID-19 patients can range from an asymptomatic state to an upper airway infection to severe pneumonia associated with acute respiratory distress syndrome (ARDS), which requires ventilatory support [2,3,4,5,6]. Chest computed tomography images of patients with the virus have shown diffuse ground-glass opacities and early-stage lymphocytopenia even before dyspnea [7], indicating the severity of the disease. The clinical spectrum of pathology presents three main phases: early infection, pulmonary involvement, and systemic hyperinflammation. The symptoms are those of a respiratory infection—cough, fatigue, and shortness of breath—as well as less commonly systemic symptoms, such as headaches, myalgia, and arthralgia [4,7]. The risk factors for the increased severity of disease progression and increased death include comorbidities, such as high blood pressure, type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease (CVD), as well as an advanced age [2,5,6].
SARS-CoV-2 is a part of the β-coronavirus genus of the Coronaviridae family, to which SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) also belong. Several members of this coronavirus family belong to α-coronavirus and β-coronavirus genera, which cause respiratory infections in humans [8,9,10,11]. The 30-kb genome of SARS-CoV-2 encodes a large auto-proteolytically non-structural protein that eventually forms the replicase–transcriptase complex. Moreover, the 3′ end of the viral genome encodes for four structural proteins, namely the spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins [9,12,13]. The SARS-CoV-2 genome shares a 79.6% sequence identity to SARS-CoV [10,14].
The crucial functional receptor for SARS-CoV-2 infection is angiotensin-converting enzyme 2 (ACE2), which belongs to the renin–angiotensin system (RAS) in humans, and it is highly expressed in the respiratory and intestinal tract [2,14,15,16,17]. SARS-CoV-2 receptor recognition is mediated by the glycosylated spike protein. After ACE2 binding, the S protein is cleaved and activated by transmembrane protease serine 2 into S1 and S2 subunits. S1 contains the receptor-binding domain, which directly binds to the peptidase domain (PD) of the ACE2 membrane, and the activated S2 subunit is responsible for membrane fusion [8,15,18,19]. Moreover, the receptor-binging domain in the S protein of SARS-CoV-2 differs in five of the six amino acid residues compared to SARS-CoV. These modifications probably explain the 10- to 20-fold higher affinity for ACE2 of SARS-CoV-2 compared with SARS-CoV [20,21].
ACE2 is part of the non-classical RAS axis [22]. ACE2 is a carboxypeptidase with two domains: a full extracellular amino-terminal PD domain and a carboxy-terminal collectrin-like domain containing a transmembrane helix intracellular segment [18,23]. The N-terminal catalytic domain of ACE2 produces angiotensin (1-7) (Ang-(1-7)) by two different processes, cleaving a residue from angiotensin I (Ang I) to produce angiotensin (1-9) (Ang-(1-9)), which has subsequent modifications made to it by other enzymes to become Ang-(1-7), and removing a single residue from angiotensin II (Ang II) to generate Ang-(1-7). Ang- (1-7) has a positive effect on different tissues because it promotes vasodilation, reduced proliferation, and prevents apoptosis [18,23].
ACE2 expresses in several tissues and organs in the body, such as the heart, kidney, small intestine, and, to a lesser extent, the lung and skeletal muscle [2,24]. It is highly expressed in the epithelium of the upper airway (nose and oropharynx), which is the principal entry point of SARS-CoV-2 in humans [7].
2. RAS Dysregulation and Its Relationship with COVID-19
RAS is a complex hormonal axis that, in physiological conditions, regulates blood pressure, hydro-electrolyte balance, inflammation, and fibrosis [25,26]. RAS is also one of the modulators of muscle mass [27,28]. RAS is divided into the following axes.
2.1. Classical Axis
This axis is composed of several peptides generated by the proteolytic action of enzymes belonging to RAS. Thus, Ang I is converted to Ang II by ACE. Ang II can bind to a family of G-protein-coupled receptors named angiotensin type 1 (AT1R) and type 2 (AT2R) receptors. The effects of AT1R-dependent Ang II and its intracellular signaling pathways result in harmful effects, such as inflammation, vasoconstriction, and atherogenesis, which can participate in the genesis of diseases, such as insulin resistance and thrombosis [29,30]. By contrast, AT2R stimulation by Ang II causes vasodilation, decreased platelet aggregation, and the promotion of insulin actions. Despite these beneficial effects, the expression of AT2R is low in most tissues in healthy adults [30] (Figure 1).
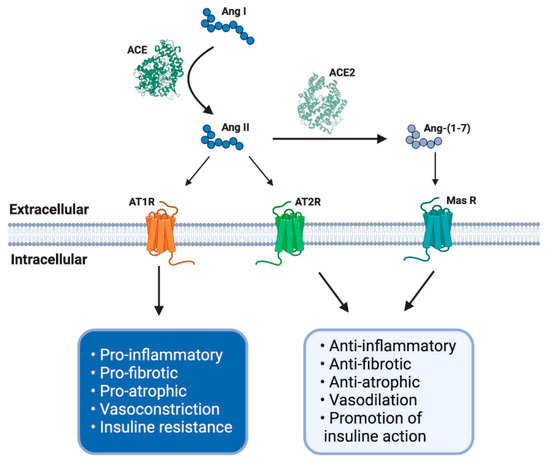
Figure 1. The renin–angiotensin system (RAS) and its physiological functions. The RAS regulates complex process as blood pressure, inflammation, carbohydrate metabolism or fibrosis, among others. It is composed of different peptides obtained by proteolytic cleavage mediated by specific enzymes belong to RAS. Thereby, angiotensin I (Ang I) is converted to Ang II by angiotensin-converting enzyme (ACE), and this second peptide can interact with its receptor angiotensin type 1 (AT1R), having some adverse biological effects, for example, an increase in blood pressure and pro-inflammatory events. However, Ang II, by its interaction with another receptor, AT2R, mediates opposite effects like vasodilatation and anti-inflammatory processes. Furthermore, Ang II can be converted to Ang-(1-7) by soluble ACE2 action and mediates the same beneficial effects through Mas receptor (MasR) signaling. ACE: angiotensin-converting enzyme; ACE2: angiotensin-converting enzyme 2; AT1R: angiotensin II type 1 receptor; AT2R: angiotensin II type 2 receptor; Ang I: angiotensin I; Ang II: angiotensin II; Ang-(1-7): angiotensin (1-7); MasR: Mas receptor; EC: extracellular; IC: intracellular. Created with BioRender.
2.2. Non-Classical Axis
The effects of Ang II in adults are regulated and, in many cases, counteracted by the non-classical RAS axis [27,31,32]. In this axis, ACE2 converts Ang II to Ang-(1-7), which has beneficial effects, such as vasodilation and anti-fibrotic and anti-atrophic effects in skeletal muscle. Ang-(1-7) signals through the Mas receptor (MasR) and promotes similar biological effects as AT2R-mediated actions [26,33] (Figure 1).
When SARS-CoV-2 enters human cells, it down-regulates the surface expression of ACE2 protein [34], which could occur due to the enzyme endocytosis complex with the virus protein S [7,35]. Furthermore, the binding of SARS-CoV-2 to ACE2 appears to induce ACE2 release as a soluble form in serum, further decreasing ACE2 activity [36,37]. These events would lead, on the one hand, to an exaggerated increase in the activation of the classical RAS pathway (ACE/Ang II/AT1R), which could induce a pro-fibrotic and pro-inflammatory state, vasoconstriction, increased membrane permeability, and apoptosis of lung epithelial cells [28,29,30,31] (Figure 2). This situation directly induces acute lung injury (ALI) and ARDS and can lead to death [10]. On the other hand, a decrease in the expression of ACE2 involves a reduction in Ang-(1-7), which could imply diminished anti-inflammatory, anti-fibrotic, and anti-atrophic effects. Both conditions, increases in Ang II/AT1R and decreases in ACE2/Ang-(1-7), have been identified in other chronic diseases, such as CVD and T2DM, and could happen in SARS-CoV-2 [18,25,38] (Figure 2).
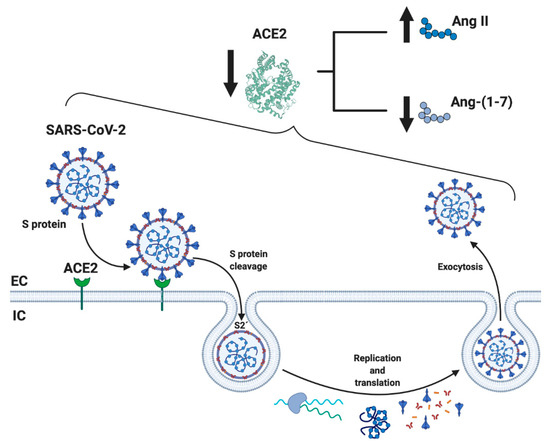
This information would indicate that the dysregulation of RAS could be fundamental in the clinical development of SARS-CoV-2 [7]. Increased activity of ACE/Ang II/AT1R has been raised as a possible cause of the pathophysiological effects of SARS-CoV. This could produce an increase in the inflammatory and fibrotic state and a decrease in ACE2/Ang-(1-7)/MasR activity, which would also happen in SARS-CoV-2 [39,40].
In this regard, it has been demonstrated that ACE2 decreases its expression in mice with severe ALI induced by acid aspiration or sepsis. Simultaneously, components of the classical RAS pathway (ACE, Ang II, AT1R) increase at the pulmonary and systemic levels. These changes promote the pathogenesis of lung disease, induce edemas, and impair lung function. The authors conclude that ACE2 has a protective effect in mice with ALI [39]. Furthermore, it has been demonstrated that the SARS-CoV spike protein increases Ang II and ACE2 down-regulation, resulting in lung injury [34]. ACE2 is upregulated through a negative feedback mechanism by blocking AT1R, leading to lung protection from virus damage, which could be attributed to the increased conversion of Ang II to Ang-(1-7) [41]. Furthermore, it has been found that ACE2 down-regulation induces the persistent elevation of Ang II through local interaction with the AT1R, triggering a vicious cycle in which Ang II down-regulates ACE2, leading to a local increase in Ang II in the tissues [42].
To date, the use of ACE and AT1R blockers (ARB) as a possible treatment to reduce lung inflammatory response and mortality in patients with COVID-19 pneumonia could confirm that RAS dysregulation is a part of the pathophysiology of COVID-19. However, this is still controversial because, for example, ARB can increase ACE2 expression, causing harmful consequences for patients with COVID-19 [43,44].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21217904
