Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
Metallic aluminum is widely used in propellants, energy-containing materials, and batteries due to its high energy density. In addition to burning in the air, aluminum can react with water to generate hydrogen. Aluminum is carbon-free and the solid-phase products can be recycled easily after the reaction. Micron aluminum powder is stable in the air and enables global trade. Aluminum metal is considered to be a viable recyclable carrier for clean energy.
- recyclable energy carrier
- aluminum fuel
- aluminum combustion
- aluminum-water reactions
- energy storage
- energy conversion system
1. Introduction
Aluminum is oxidized in air (oxygen), water, and carbon dioxide. The studies on Al-CO2 are mainly conducted in deep space [47,48]. Therefore, this text focuses on the mechanism and application of aluminum-air (oxygen) reaction and aluminum-water reaction. Aluminum fuel is applied in a wide variety of situations, such as underwater propellants, rocket propellants, explosives, fireworks, and batteries [37,44]. Hydrogen and heat are generated during the reaction of aluminum with water [1,10]. Hydrogen is frequently used as fuel for engines and fuel cells, and propellants for rockets and underwater vehicles commonly use the heat generated by the reaction [37,44]. Normally, propellants need to react at high temperatures (T > 3000 K) [37]. Currently, aluminum-water reactions for the preparation of hydrogen are generally performed at low temperatures (below the boiling point of water) [44]. The low-temperature reaction only utilizes the chemical energy in the hydrogen and the heat is seriously wasted. Propellants typically just utilize the exothermic heat of the reaction and the hydrogen produced is vented outward to generate power. Chemical energy is wasted in aluminum. Bai et al. [49] proposed an energy storage system based on aluminum-air reaction. The system has been thermodynamically evaluated by Aspen Plus and compared with other systems. The results show that aluminum-fueled energy storage systems have a higher roundtrip efficiency and that the cost of electricity from aluminum-fueled energy storage is comparable to that of coal-fired power plants.
This work focuses on energy conversion systems that allow the full exploitation of the chemical energy in aluminum-based fuel. Zero-carbon energy conversion systems at scale require high energy and power densities to enable the supply of power to households, industry and equipment, remote power sites, grids, etc. [1]. Similar to conventional hydrocarbon fuels, the combustion of aluminum powder at high temperature and pressure is required for the system to obtain high energy and power density. Depending on the oxidizer, aluminum-fuel-based energy conversion systems are classified into the following two pathways, as shown in Figure 1 [1,49].
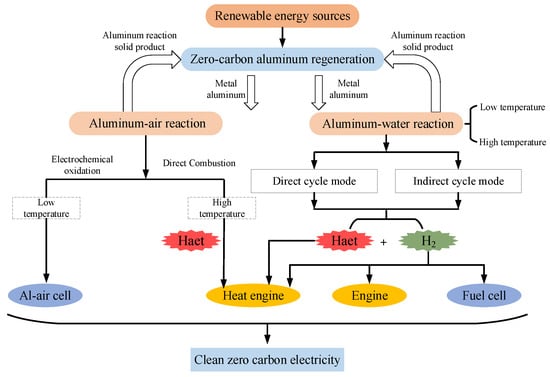
Figure 1. Schematic diagram of the zero-carbon-cycle power generation system based on aluminum fuel.
- (1)
-
Aluminum-air reaction: Similar to fossil fuels, aluminum powder is combusted directly in air, releasing heat to fuel heat engines or for use in fuel cells.
- (2)
-
Aluminum-water reaction: Hydrogen and heat are obtained during the aluminum-water reaction, which allows its application in engines, heat engines, and fuel cells [45].
The two routes are disclosed in Figure 1. Each of these loops shows two paths. The aluminum-air reaction has both high- and low-temperature pathways. One route is the aluminum-air electrochemical reaction inside the cell at low temperatures. The other route is the combustion of aluminum powder in air at high temperatures, which has an energy density higher than that of the low-temperature route. Aluminum has a high chemical equivalent value, 2.98 Ah·g−1, second only to lithium [50,51]. Aluminum-air batteries convert the chemical energy of aluminum directly into electrical energy [52]. Aluminum-air batteries mainly consist of an aluminum anode, electrolyte, and air electrode [51,53]. Aluminum-air batteries are environment-friendly in operation and also have the advantages of high specific energy, low mass, and low noise [52,54]. Aluminum-air batteries are highly adaptable and stable, but they have relatively low power, low electrochemical efficiency, and high self-discharge rates [55]. In addition to technical challenges, the high cost of electricity for aluminum-air batteries also restricts its large-scale application [56]. Numerous studies have concluded that aluminum-air batteries have great potential and advantages for applications such as underwater power supplies, electric vehicles, and power supply stations [55,57,58]. Electrochemical oxidation is also one of the pathways for the realization of aluminum as the energy carrier.
The aluminum-water reaction also occurs in both high- and low-temperature modes. The hydrogen and heat from the reaction are commonly used in various turbines and fuel cells [45]. Whatever the route in an aluminum-fuel-based energy conversion system, clean renewable primary energy is converted into the chemical energy in aluminum. Aluminum is easy to store and transport, with the subsequent application of aluminum fuel as required [37]. The solid products of the reaction are collected and can be regenerated into aluminum, which continues to participate in the system cycle. Regarding aluminum powder particle size, there are problems of incomplete combustion and loss of combustion efficiency with micron particles [1]. Nanometer aluminum powder avoids such problems [59]. However, nanoparticles are expensive, their energy recycling is low, and they have safety issues. Nanoparticles and its oxides may cause wear problems, and the separation as well as collection of both is difficult. In contrast, micron-sized aluminum powders offer higher energy density, cycle efficiency, and safety, and are less expensive.
2. Aluminum-Air Reaction Power Generation
Aluminum combustion in air directly provides the system with high power generation efficiency and energy density [37]. The combustion reaction equation is indicated in Equation (1).
4Al+3O2=2Al2O3
The metal–air reaction is expected to apply in internal combustion engines to attain high utilization efficiency [1]. The same is true for aluminum powder. The development of specialized metal combustors for burning metals increases the viability and reliability of the system and is one of the pivotal points of current research. Bergthorson [10] proposed a principle for the utilization of systems based on the metal–air reaction, including a cyclone combustor that is usable for metals. This metal cyclone burner mainly consists of a metal fuel storage tank, a metal fuel combustion chamber, a combustion product cyclone separator, and a combustion product storage tank. The metal fuels are violently oxidized by the air in the combustion chamber. The solid products created by the reaction are separated and recovered. The high-temperature gas streams produced by combustion can be used in a variety of scenarios for power and heat generation at various scales.
For aluminum, the aluminum-air-based energy conversion system has three stages, the energy storage stage, the energy release stage, and the regeneration stage, as indicated in Figure 2. The energy storage section powers the process of aluminum electrolysis through renewable energy sources [23]. In the energy storage stage, renewable energy is used to power the electrolytic aluminum process. In the energy release process, the aluminum obtained by electrolysis is passed through a combustion power cycle to generate electricity. The chemical energy in aluminum is converted into electricity for transmission to the outside world. The solid-phase products from the combustion of the aluminum powder are also collected. In the regeneration process, the collected solid combustion products are re-electrolyzed into aluminum, which enables the regeneration process of aluminum along with the energy storage process [23]. The aluminum fuel is recycled throughout the process with virtually no consumption. The aluminum-air combustion power generation system is still in the laboratory research period. The research on the aluminum-air combustion mechanism and reaction kinetics, combustor design and development, combustion product morphology and separation, and high-temperature gas heat transfer should be given more attention. Aluminum powder has similar physical properties to solid fossil fuels. If coal-fired power plants are retrofitted for metal fuel combustion, this can significantly reduce infrastructure investment and contribute to zero carbon electricity generation.
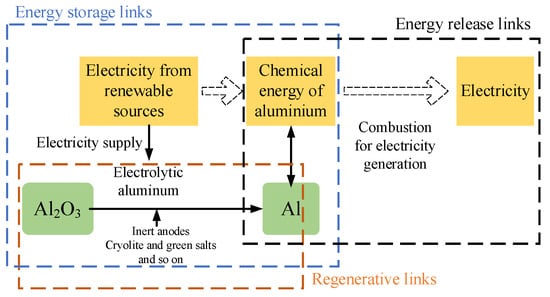
Figure 2. Schematic diagram of the energy conversion principle based on aluminum-air reaction [23].
3. Aluminum-Water Reaction Power Generation
The heat generated from the oxidation of metals with water is fully utilized at high temperatures. Otherwise, fuel cells are needed to increase the chemical energy of the hydrogen [12]. The stoichiometric ratio and exotherm of the reaction between aluminum and water vary at various temperatures, and the high-temperature aluminum-water reaction is illustrated in Equation (2).
2Al(s)+3H2O(g)→Al2O3(s)+H2(g)
Aluminum-water reaction energy conversion systems are divided into indirect circulation systems and direct circulation systems depending on the reaction cycle [49]. The heat generated by the reaction is coupled to the power generation process by the heat exchanger in indirect-cycle systems, and the reaction process is coupled to the power generation process directly in direct-cycle systems. Indirect-cycle systems approximate coal-fired power generation and direct-cycle systems approximate gas turbine power generation. The principle of the indirect circulation system based on the steam Rankine cycle and the aluminum-water reaction is presented in Figure 3a and the principle of the direct circulation system based on the aluminum-water reaction is displayed in Figure 3b.
(1) Indirect circulation system
As illustrated in Figure 3a, aluminum powder is oxidized by excess water in the aluminum-water reactor to ensure complete and adequate aluminum-water reaction. The heat generated from the reaction is employed to heat the working medium in the steam Rankine cycle. Then, the high-temperature and high-pressure workpiece flows into the steam turbine to do the work and drive the generator to generate electricity. The aluminum-water reaction products include both gas-phase and solid-phase products. The solid-phase products are recovered by collection and then used as feedstock for the alumina electrolysis process in the next energy storage cycle. The gas-phase product is mainly the mixture of high-temperature steam and hydrogen, which passes into the condenser after heat exchange with the circulating working medium in the reactor. The water vapor in the gas-phase mixture is separated from the hydrogen by condensation in the condenser, thus obtaining water and hydrogen. The water separated from the condenser is filtered and then continues to participate in the system cycle. The electricity and hydrogen produced by the system are utilized according to demand.
Montorsi et al. [44,60] conducted further research on combustion devices, system construction, and optimization of indirect-cycle systems for aluminum-water reactions. The thermal characteristics of four different circulation systems were analyzed by the combination of aggregated and distributed parameters. The influences of combustion temperature, water-to-aluminum ratio, and combustion pressure on the power generation efficiency, system efficiency, and hydrogen production rate of the cycle system were investigated. It was found that variations in parameters such as combustion temperature, water-to-aluminum ratio, and combustion pressure have effects on the efficiency of the system and have little effect on the hydrogen yield. Yang et al. [61] established an indirect-cycle system based on the aluminum-water reaction and analyzed the influence of key parameters such as cell conversion efficiency through systematic simulations. The system power generation efficiency was 49.25%, improving the battery conversion efficiency, which is beneficial to the utilization efficiency of the system. For aluminum-water-reaction indirect-cycle power generation systems, aluminum-water reaction kinetics, aluminum-water reactor design, and system design all require further research.
(2) Direct circulation system
As indicated in Figure 3b, the high-temperature and high-pressure gas mixture from the aluminum-water reaction in the direct-cycle system flows directly into the hydrogen-steam turbine to do the work and drive the generator to produce electricity. Compared to the aluminum-water reactor in an indirect circulation system, the aluminum-water reactor in a direct circulation system has to be at high temperature and pressure, which places higher demands on the equipment. The aluminum-water reactor is the critical equipment in the direct circulation system. Turbines and fuel cells are also used to harness the mixture of gases generated by the aluminum-water reaction [1,12,62].
The internal combustion engine enables clean and efficient utilization of the high-temperature and -pressure hydrogen from the reaction. Interception of the solid-phase products after the reaction is required in order to avoid wearing out the generator. In addition to internal combustion engines, external combustion engines are also available for direct circulation systems, a system in which a hydrogen-steam mixture is combusted together with air with high efficiency [1]. Research on aluminum-water combustion direct-cycle systems has focused on aluminum-water reaction characteristics at high pressure, aluminum fuel delivery devices, cycle system construction, and system thermal characteristics.
Vlaskin et al. [62] constructed an experimental hydrogen cogeneration plant based on an aluminum-water reaction. The average reaction temperature and pressure of the system were 324 °C and 15 MPa, respectively. The electrical efficiency of the power plant was 12% and the total efficiency was 72%. Vlaskin et al. [63] also found that the efficiency of power plants was improved by utilizing the mixture of gases from the aluminum-water reaction via a gas turbine. Based on the aluminum-water reaction, Yang et al. [61] constructed the direct-cycle co-generation system. The system produced approximately 22.2 MJ·kg(Al)−1 of heat and electricity with a power generation efficiency of 41.52%. Franzoni et al. [12] calculated the energy conversion efficiency of the aluminum-water reaction system when superheated steam cycles and combined heat and power cycles were used. Hydrogen from the aluminum-water reaction for fuel cells had system efficiencies in the range of 0.62–0.85. In contrast to aluminum-air energy conversion systems, aluminum-water reaction energy conversion systems produce and utilize hydrogen. The increasing complexity of the system has the potential to reduce its efficiency [37]. The aluminum regeneration process is the critical link for aluminum as a recyclable energy carrier. Aluminum electrolysis is a high-energy-consuming industry, and the electricity consumption in the aluminum regeneration process is significantly influencing the economy of the aluminum-based fuel energy conversion system. If the electricity generated by new energy is utilized for aluminum regeneration, not only is the volatility of new energy generation alleviated, but the cost of electricity consumption of the system is also reduced. The economy of the system is improved. For the aluminum-water reaction direct circulation system, the relevant work is still mainly in the basic research of aluminum-water high-pressure combustion in the laboratory, the construction and optimization of the circulation system, and the analysis of the system economy [12]. Aluminum-water high-pressure combustion reaction kinetics, aluminum-water combustion reactor development, the system key equipment design, and large-scale system integration still need further in-depth research to achieve engineering applications.
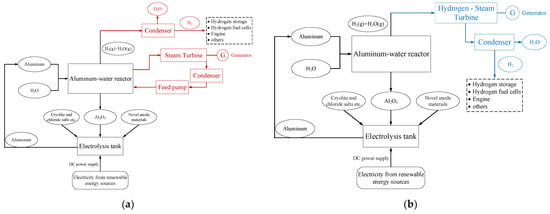
Figure 7. Schematic diagram of the energy conversion principle based on the aluminum-water reaction [64]. (a) Indirect circulatory system. (b) Direct circulation system.
This entry is adapted from the peer-reviewed paper 10.3390/en16010436
This entry is offline, you can click here to edit this entry!
