Fruiting bodies, mycelia, or spores in the form of extracts or powder of various medicinal mushrooms are used to prevent, treat, or cure a range of ailments and balance a healthy diet. Medicinal mushrooms are found in several genera of fungi and their fruit bodies, cultured mycelia, and cultured broth contains phytochemical constituents such as triterpenes, lectins, steroids, phenols, polyphenols, lactones, statins, alkaloids, and antibiotics. Edible mushrooms are considered functional foods that can be used as supplements for complementary and alternative medicines where the markets are growing rapidly. Several species of edible mushrooms possess therapeutic potential and functional characteristics. The psilocybin-containing types, sometimes known as magic mushrooms, have been utilized for generations by indigenous communities due to their hallucinogenic, medicinal, and mind-manifestation properties.
- mycology
- psilocybin
- psychiatric disorders
- psychedelic mushrooms
1. Medicinal Mushrooms
|
Mushrooms |
Uses |
Bioactive Constituents |
References |
|---|---|---|---|
|
White button mushroom (Agaricus bisporus), Almond mushroom (A. subrufescens), Caterpillar fungus (Cordyceps sinensis), Shaggy ink cap (Coprinus comatus), Lingzhi (Ganoderma lucidum), White rot fungus/Chaga (Inonotus obliquus), Phellinus linteus, Oyster mushroom (Pleurotus spp.), Poria cocos, and Sparassis crispa |
Possessed hypoglycemic effects on reducing blood glucose levels and antidiabetic effects. |
Dietary fiber along with the polyphenols, vitamin C, and ergothioneine, as well as proteins, and polysaccharides (β-Glucans and oligosaccharides). |
|
|
Almond mushroom |
Induced apoptosis of intestinal cancers. |
Soluble fibers serve as a desirable food source for bacteria that generate short-chain fatty acids such as butyrate, which may be able to stimulate apoptosis of cancers in human intestinal. |
[9] |
|
Lingzhi |
Effective for treatment of gastric cancer. |
Treatment of a gastric cancer cell line with methanolic extract resulted in an increase in cellular autophagy and the production of autophagosomes (AGS). |
|
|
Himematsutake (A. blazei) |
Traditional medicine from Japan and used for treatments of diabetes, hyperlipidemia, arteriosclerosis, and chronic hepatitis. In animal study, it increased proliferation of monocyte and promoted destruction of cells with DNA alterations that correlate with the development of cancer. |
The immune system is modulated by bioactive β-glucans in supplemental diets and is rendered more effective with regard to phagocytic activity. |
[9] |
|
Trametes versicolor |
The medicinal mushroom frequently used in traditional Chinese medicine for its antiviral, antitumor, and immunomodulatory effects. |
The polysaccharide Krestin (PSK) is commercially available for use in cancer immunotherapy. |
[12] |
|
Flammulina velutipes, Pholiota spp., Lingzhi and straw mushroom (Volvariella volvacea) |
The immunomodulatory proteins had been isolated from the fruiting body and cultured mycelia of the mushrooms. |
Lectins can bind to cell surface carbohydrates, agglutinate cells, and inhibit cancer cell growth. |
|
|
Chaga (Inonotus obliquus) |
A fungal parasite, grows on birch trees in colder northern climates with anticancer properties. |
Betulin or betulinic acid isolate illustrates platelet adhesion and aggregation plays an important role in the pathogenesis of thrombosis, particularly arteriothrombosis. |
[16] |
|
Cordyceps spp. |
The mushrooms are used in Traditional Chinese medicine due to their various therapeutic properties including immunoregulative, anticancer, antibacterial, and antifungal activities. |
Cordycepin, ophicordin, polysaccharides, and L-tryptophan are bioactive constituents isolated from the Cordyceps such as C. militaris, also known as north Cordyceps. |
2. Medicinal Mushroom Diversity and Taxonomy Characteristics of Magic Mushrooms
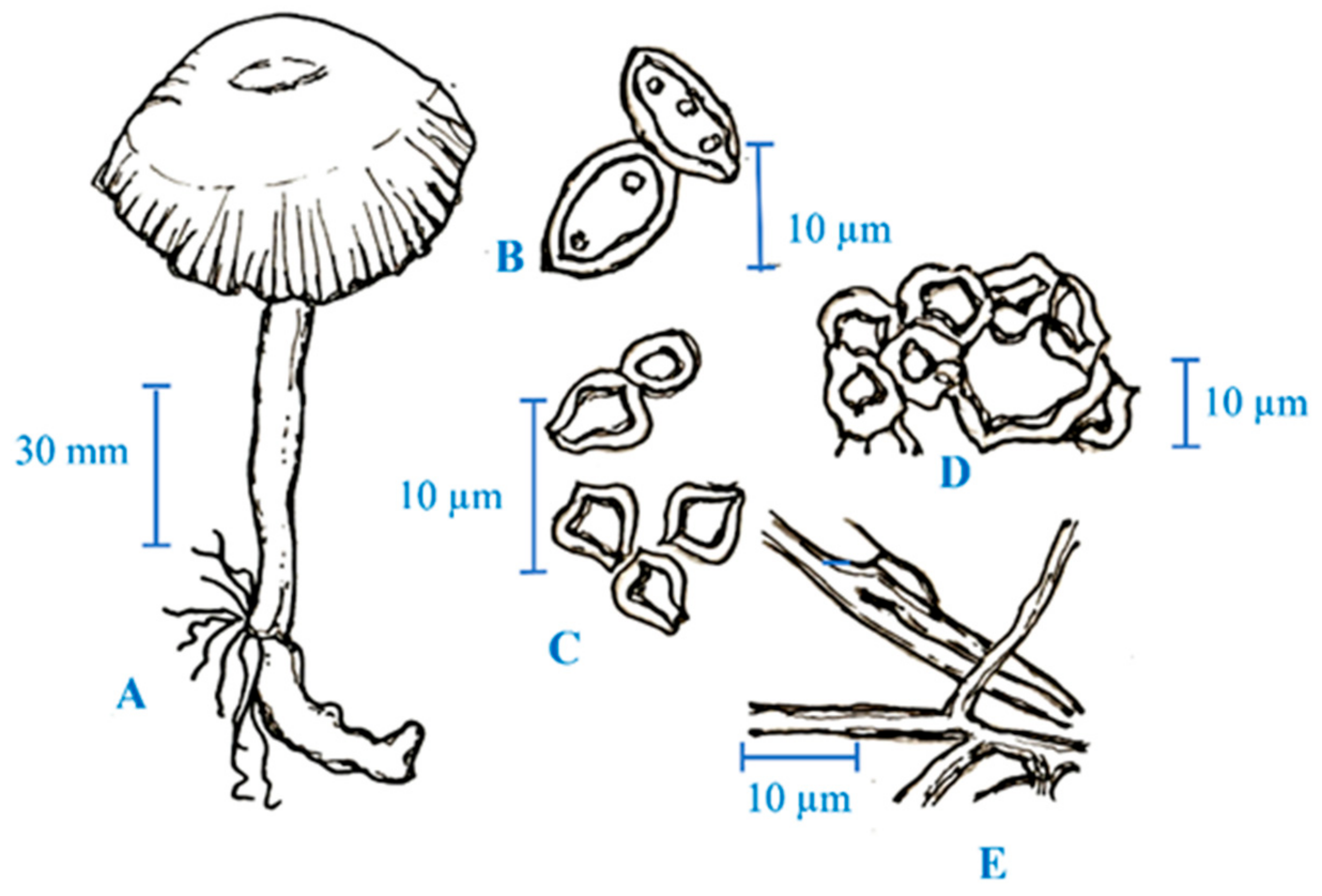
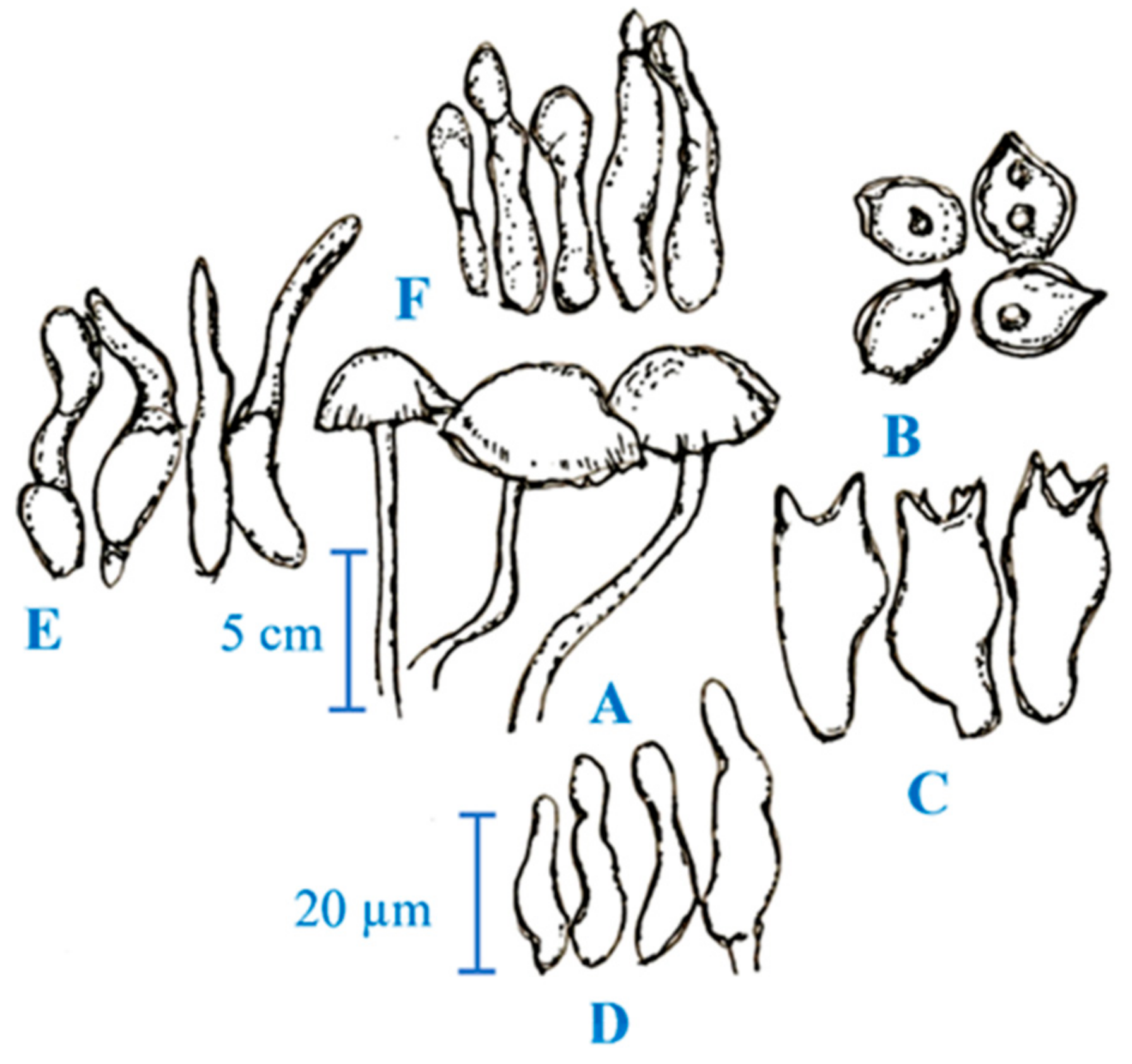
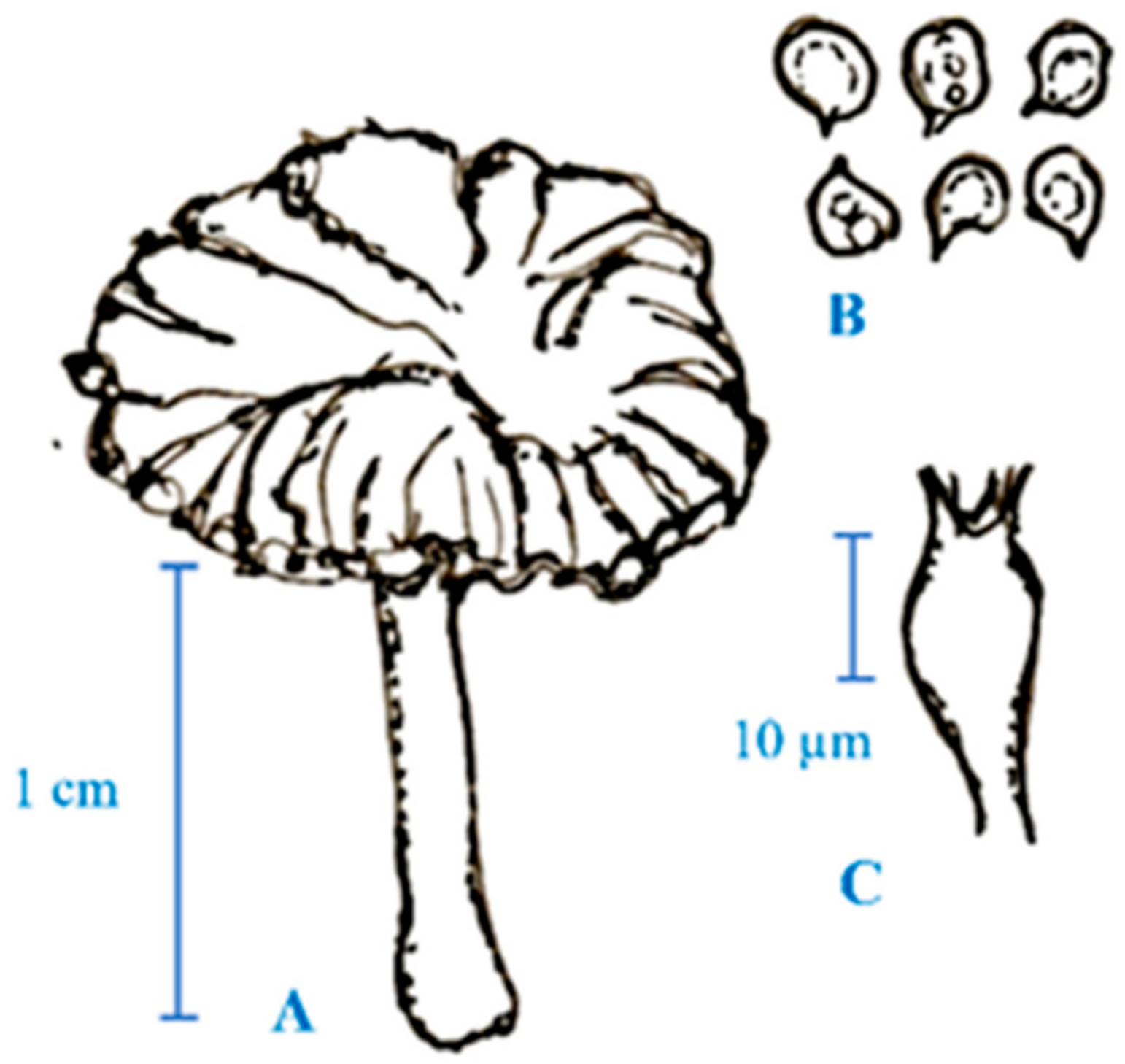
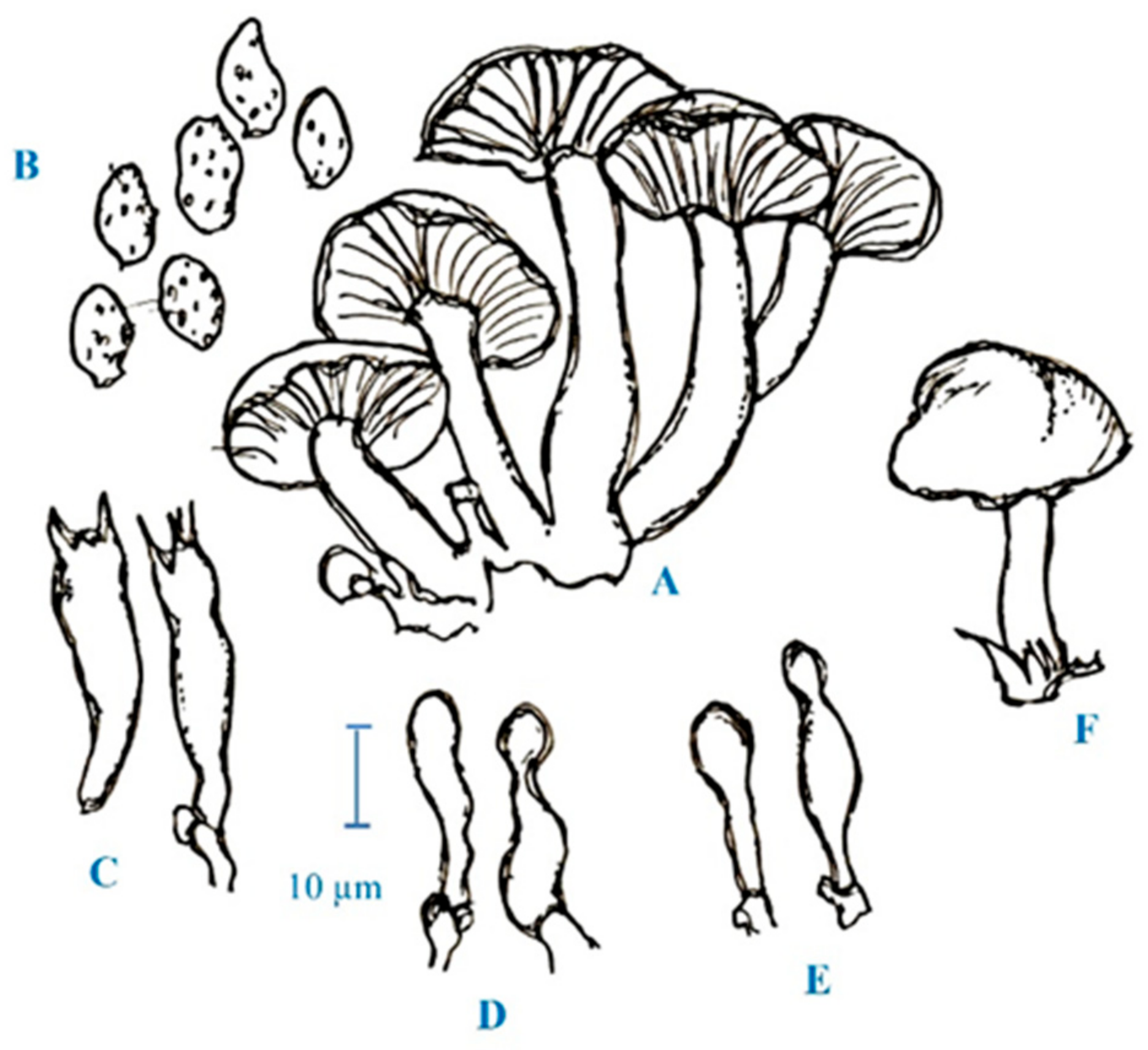
3. Active Ingredients and Medicinal Properties
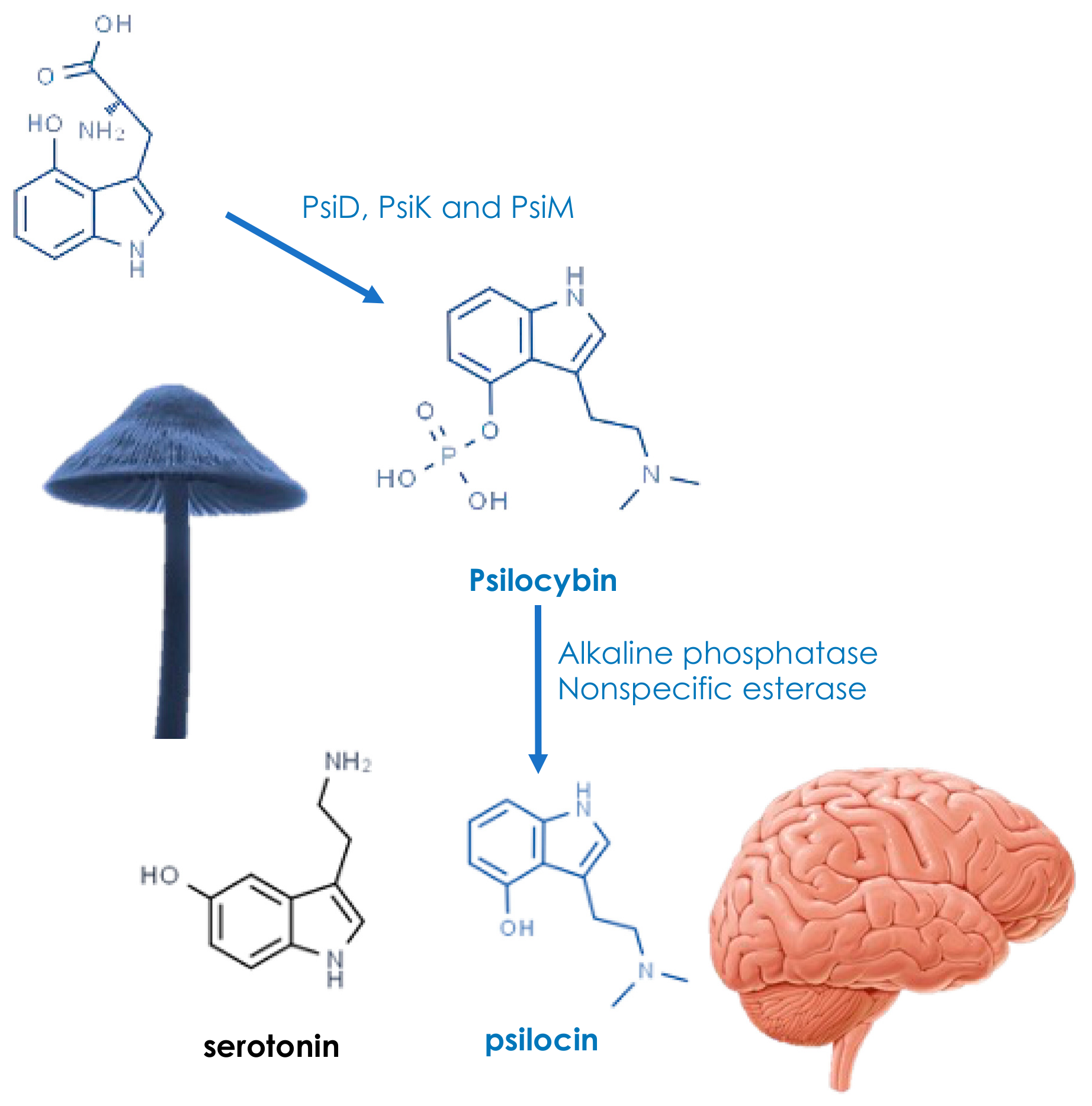
This entry is adapted from the peer-reviewed paper 10.3390/agronomy12123185
References
- Elkhateeb, W.A. What medicinal mushroom can do. Chem. Res. J. 2020, 5, 106–118.
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356.
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2017, 151, 548–565.
- Chang, S.T.; Wasser, S.P. The cultivation and environmental impact of mushrooms. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK, 2017.
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal mushrooms in prevention and control of diabetes mellitus. Fungal Divers. 2012, 56, 1–29.
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56.
- Volman, J.; Mensink, R.; Van Griensven, L.; Plat, J. Effects of α-glucans from Agaricus bisporus on ex vivo cytokine production by LPS and PHA-stimulated PBMCs; a placebo-controlled study in slightly hypercholesterolemic subjects. Eur. J. Clin. Nutr. 2010, 64, 720–726.
- Kim, Y.-W.; Kim, K.-H.; Choi, H.-J.; Lee, D.-S. Anti-diabetic activity of β-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol. Lett. 2005, 27, 483–487.
- Ishii, P.L.; Prado, C.K.; Mauro, M.d.O.; Carreira, C.M.; Mantovani, M.S.; Ribeiro, L.R.; Dichi, J.B.; Oliveira, R.J. Evaluation of Agaricus blazei in vivo for antigenotoxic, anticarcinogenic, phagocytic and immunomodulatory activities. Regul. Toxicol. Pharmacol. 2011, 59, 412–422.
- Rony, K.; Mathew, J.; Neenu, P.; Janardhanan, K. Ganoderma lucidum (Fr.) P. Karst occurring in South India attenuates gastric ulceration in rats. Indian J. Nat. Prod. Resour. 2011, 2, 19–27.
- Reis, F.S.; Lima, R.T.; Morales, P.; Ferreira, I.C.; Vasconcelos, M.H. Methanolic extract of Ganoderma lucidum induces autophagy of AGS human gastric tumor cells. Molecules 2015, 20, 17872–17882.
- Li, F.; Wen, H.; Zhang, Y.; Aa, M.; Liu, X. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Sci. China Life Sci. 2011, 54, 379–385.
- She, Q.-B.; Ng, T.-B.; Liu, W.-K. A Novel Lectin with Potent Immunomodulatory Activity Isolated from Both Fruiting Bodies and Cultured Mycelia of the Edible Mushroom Volvariella volvacea. Biochem. Biophys. Res. Commun. 1998, 247, 106–111.
- Sze, S.; Ho, J.; Liu, W. Volvariella volvacea lectin activates mouse T lymphocytes by a calcium dependent pathway. J. Cell. Biochem. 2004, 92, 1193–1202.
- Zhang, G.; Sun, J.; Wang, H.; Ng, T. A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim. Pol. 2009, 56, 415–421.
- Hyun, K.W.; Jeong, S.C.; Lee, D.H.; Park, J.S.; Lee, J.S. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006, 27, 1173–1178.
- Cui, L.; Dong, M.S.; Chen, X.H.; Jiang, M.; Lv, X.; Yan, G. A novel fibrinolytic enzyme from Cordyceps militaris, a Chinese traditional medicinal mushroom. World J. Microbiol. Biotechnol. 2008, 24, 483–489.
- Zhou, X.; Meyer, C.U.; Schmidtke, P.; Zepp, F. Effect of cordycepin on interleukin-10 production of human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2002, 453, 309–317.
- Nkadimeng, S.M.; Nabatanzi, A.; Steinmann, C.M.L.; Eloff, J.N. Phytochemical, Cytotoxicity, Antioxidant and Anti-Inflammatory Effects of Psilocybe Natalensis Magic Mushroom. Plants 2020, 9, 1127.
- Gartz, J.; Wiedemann, G. Discovery of a new caerulescent Psilocybe mushroom in Germany: Psilocybe germanica sp.nov. Drug Test Anal. 2015, 7, 853–857.
- Shaw, L.; Rea, K.; Lachowsky, N.J.; Roth, E.A. Magic Mushroom Use: A Qualitative Interview Study of Post-Trip Impacts and Strategies for Optimizing Experiences. J. Psychoact. Drugs 2022, 1–8.
- Amsterdam, J.v.; Opperhuizen, A.; Brink, W.v.d. Harm potential of magic mushroom use: A review. Regul. Toxicol. Pharmacol. 2011, 59, 423–429.
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332.
- Ganeshpurkar, A.; Rai, G.; Jain, A.P. Medicinal mushrooms: Towards a new horizon. Pharm. Rev. 2010, 4, 127–135.
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and Taxane Production by Taxomyces andreanae, an Endophytic Fungus of Pacific Yew. Science 1993, 260, 214–216.
- Rahi, D.K.; Rahi, S.; Pandey, A.; Rajak, R. Enzymes from mushrooms and their industrial application. In Advances in Fungal Biotechnology; I. K. International: New Delhi, India, 2009; pp. 136–184.
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803.
- Egli, S. Mycorrhizal mushroom diversity and productivity—An indicator of forest health? Ann. For. Sci. 2011, 68, 81–88.
- Le Tacon, F.; Rubini, A.; Murat, C.; Riccioni, C.; Robin, C.; Belfiori, B.; Zeller, B.; De la Varga, H.; Akroume, E.; Deveau, A.; et al. Certainties and uncertainties about the life cycle of the Périgord black truffle (Tuber melanosporum Vittad.). Ann. For. Sci. 2016, 73, 105–117.
- Yang, X.; Luedeling, E.; Chen, G.; Hyde, K.D.; Yang, Y.; Zhou, D.; Xu, J.; Yang, Y. Climate change effects fruiting of the prize matsutake mushroom in China. Fungal Divers. 2012, 56, 189–198.
- Stamets, P. Growing Gourmet and Medicinal Mushrooms; Ten Speed Press: Berkeley, CA, USA, 2011.
- Strauss, D.; Ghosh, S.; Murray, Z.; Gryzenhout, M. An Overview on the Taxonomy, Phylogenetics and Ecology of the Psychedelic Genera Psilocybe, Panaeolus, Pluteus and Gymnopilus. Front. For. Glob. Chang. 2022, 5, 813998.
- Lenz, C.; Wick, J.; Braga, D.; García-Altares, M.; Lackner, G.; Hertweck, C.; Gressler, M.; Hoffmeister, D. Injury-Triggered Blueing Reactions of Psilocybe “Magic” Mushrooms. Angew. Chem. Int. Ed. 2020, 59, 1450–1454.
- Melgarejo-Estrada, E.; Suarez, M.E.; Rocabado, D.; Maillard, O.; Lechner, B.E. Checklist of Bolivian Agaricales: 1. Species with dark and pink spore prints. Mycotaxon 2020, 134, 739.
- Van Court, R.C.; Wiseman, M.S.; Meyer, K.W.; Ballhorn, D.J.; Amses, K.R.; Slot, J.C.; Dentinger, B.T.M.; Garibay-Orijel, R.; Uehling, J.K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319.
- Guzmán, G.; Ramírez Guillén, F.; Hyde, K.D.; Karunarathna, S.C. Psilocybe sp. in Thailand: Four new species and a review of previously recorded species. Mycotaxon 2012, 119, 65–81.
- Gerhardt, E. Taxonomische Revision der Gattungen Panaeolus and-Panaeolina (Fungi, Agaricales, Coprinaceae); Schweizerbart and Borntraeger Science Publishers: Stuttgart, Germany, 1996.
- He, M.-Q.; Zhao, R.-L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.; Raspe, O.; Kakishima, M.; Sanchez-Ramirez, S. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367.
- Ediriweera, S.; Wijesundera, R.; Nanayakkara, C.; Weerasena, J. First report of Panaeolus sphinctrinus and Panaeolus foenisecii (Psathyrellaceae, Agaricales) on elephant dung from Sri Lanka. Front. Environ. Microbiol. 2015, 1, 19–23.
- Amandeep, K.; Atri, N.; Munruchi, K. Two new coprophilous varieties of Panaeolus (Psathyrellaceae, Agaricales) from Punjab, India. Mycosphere 2013, 4, 616–625.
- Amandeep, K.; Atri, N.; Munruchi, K. Two new species of Panaeolus (Psathyrellaceae, Agaricales) from coprophilous habitats of Punjab, India. J. New Biol. Rep. 2014, 3, 125–132.
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Effects and safety of Psilocybe cubensis and Panaeolus cyanescens magic mushroom extracts on endothelin-1-induced hypertrophy and cell injury in cardiomyocytes. Sci. Rep. 2020, 10, 22314.
- Iliffe, R. Getting to grips with pluteus. Field Mycol. 2010, 11, 78–92.
- Hosen, M.I.; Liang, X.; Xu, J.; Li, T.H. Pluteus squarrosus sp. nov.(Pluteus sect. Celluloderma, Pluteaceae) from northeast China. Nord. J. Bot. 2019, 37.
- Stijve, T.; Bonnard, J. Psilocybine et urée dans le genre Pluteus. Mycol. Helv. 1986, 2, 123–130.
- Justo, A.; Vizzini, A.; Minnis, A.M.; Menolli, N.; Capelari, M.; Rodríguez, O.; Malysheva, E.; Contu, M.; Ghignone, S.; Hibbett, D.S. Phylogeny of the Pluteaceae (Agaricales, Basidiomycota): Taxonomy and character evolution. Fungal Biol. 2011, 115, 1–20.
- Gartz, J. Magic Mushrooms Around the World: A Scientific Journey Across Cultures and Time; LIS Publications: Los Angeles, CA, USA, 1996.
- Stamets, P. Psilocybin Mushrooms of the World; Ten Speed Press: Berkeley, CA, USA, 1996.
- Holec, J. The Genus Gymnopilus (Fungi, Agaricales) in the Czech Republic with Respect to Collections from Other European Countries, Acta Musei Natl. Pragae 2005, 61, 1–52.
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 334.
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Non-volatile components of several medicinal mushrooms. Food Res. Int. 2001, 34, 521–526.
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Antioxidant Properties of Several Medicinal Mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077.
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80.
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381.
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880.
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678.
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT-Food Sci. Technol. 2014, 59, 689–694.
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269.
- Johnson, M.W.; Griffiths, R.R. Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 2017, 14, 734–740.
- Fricke, J.; Blei, F.; Hoffmeister, D. Enzymatic Synthesis of Psilocybin. Angew. Chem. Int. Ed. 2017, 56, 12352–12355.
- Kalberer, F.; Kreis, W.; Rutschmann, J. The fate of psilocin in the rat. Biochem. Pharmacol. 1962, 11, 261–269.
- Wolbach, A.; Miner, E.; Isbell, H. Comparison of psilocin with psilocybin, mescaline and LSD-25. Psychopharmacologia 1962, 3, 219–223.
- Tylš, F.; Páleníček, T.; Horáček, J. Psilocybin–summary of knowledge and new perspectives. Eur. Neuropsychopharmacol. 2014, 24, 342–356.
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143.
- Winter, J.; Rice, K.; Amorosi, D.; Rabin, R. Psilocybin-induced stimulus control in the rat. Pharmacol. Biochem. Behav. 2007, 87, 472–480.
- Fantegrossi, W.; Woods, J.; Winger, G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav. Pharmacol. 2004, 15, 149–157.
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197.
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627.
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740.
- Schindler, E.A.; Gottschalk, C.H.; Weil, M.J.; Shapiro, R.E.; Wright, D.A.; Sewell, R.A. Indoleamine hallucinogens in cluster headache: Results of the clusterbusters medication use survey. J. Psychoact. Drugs 2015, 47, 372–381.
- Nichols, D.E.; Frescas, S. Improvements to the synthesis of psilocybin and a facile method for preparing the O-acetyl prodrug of psilocin. Synthesis 1999, 1999, 935–938.
- Nichols, D.E. Psilocybin: From ancient magic to modern medicine. J. Antibiot. 2020, 73, 679–686.
- Fricke, J.; Lenz, C.; Wick, J.; Blei, F.; Hoffmeister, D. Production Options for Psilocybin: Making of the Magic. Chem. Eur. J. 2019, 25, 897–903.
