Postoperative pancreatic fistula (POPF) is a troublesome complication after pancreatic surgeries, and grade C POPF is the most serious situation among pancreatic fistulas. The incidence of grade C POPF varies from less than 1% to greater than 9%, with an extremely high postoperative mortality rate of 25.7%. The patients with grade C POPF finally undergo surgery with a poor prognosis after various failed conservative treatments. Although various surgical and perioperative attempts have been made to reduce the incidence of grade C POPF, the rates of this costly complication have not been significantly diminished. Hearteningly, several related studies have found that intra-abdominal infection from intestinal flora could promote the development of grade C POPF, which would help physicians to better prevent this complication.
- grade C postoperative pancreatic fistula
- operation techniques
- treatment
1. Introduction
2. Risk Factors for Grade C POPF
3. Operation Technique
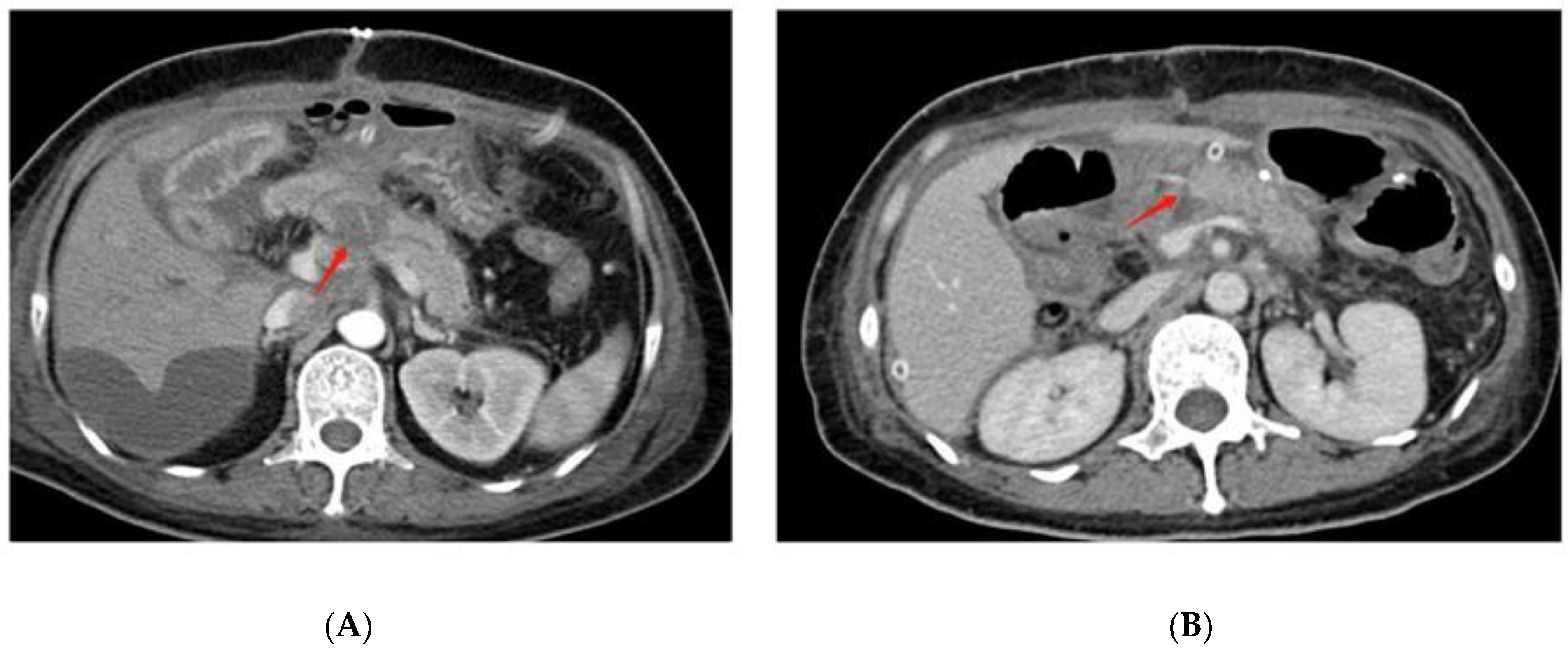
3.1. Pancreaticojejunostomy Dehiscence Is Found in Grade C POPF after PD on Radiographic Examination
3.2. Reconstruction Methods
3.3. Anastomotic Techniques
3.4. Stent or No-Stent
3.5. Two-Stage Surgical Procedure
4. Surgical Treatment for Grade C POPF
4.1. Debridement and Open Drainage
4.2. Completion Pancreatectomy
4.3. Revision of the Pancreatic Anastomosis
4.4. The Bridge Stent Technique
This entry is adapted from the peer-reviewed paper 10.3390/jcm11247516
References
- Kleespies, A.; Albertsmeier, M.; Obeidat, F.; Seeliger, H.; Jauch, K.-W.; Bruns, C.J. The challenge of pancreatic anastomosis. Langenbeck’s Arch. Surg. 2008, 393, 459–471.
- Zhang, H.; Zhu, F.; Shen, M.; Tian, R.; Shi, C.J.; Wang, X.; Jiang, J.X.; Hu, J.; Wang, M.; Qin, R.Y. Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br. J. Surg. 2015, 102, 4–15.
- Lubrano, J.; Bachelier, P.; Paye, F.; Le Treut, Y.P.; Chiche, L.; Sa-Cunha, A.; Turrini, O.; Menahem, B.; Launoy, G.; Delpero, J.-R. Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur. J. Surg. Oncol. 2018, 44, 1078–1082.
- Yekebas, E.F.; Wolfram, L.; Cataldegirmen, G.; Habermann, C.R.; Bogoevski, D.; Koenig, A.M.; Kaifi, J.; Schurr, P.G.; Bubenheim, M.; Nolte-Ernsting, C.; et al. Postpancreatectomy hemorrhage: Diagnosis and treatment: An analysis in 1669 consecutive pancreatic resections. Ann. Surg. 2007, 246, 269–280.
- Feng, J.; Chen, Y.-L.; Dong, J.-H.; Chen, M.-Y.; Cai, S.-W.; Huang, Z.-Q. Post-pancreaticoduodenectomy hemorrhage risk factors, managements and outcomes. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 513–522.
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery 2005, 138, 8–13.
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Hilal, M.A.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591.
- Fu, S.-J.; Shen, S.-L.; Li, S.-Q.; Hu, W.-J.; Hua, Y.-P.; Kuang, M.; Liang, L.-J.; Peng, B.-G. Risk factors and outcomes of postoperative pancreatic fistula after pancreatico-duodenectomy: An audit of 532 consecutive cases. BMC Surg. 2015, 15, 34.
- Fuks, D.; Piessen, G.; Huet, E.; Tavernier, M.; Zerbib, P.; Michot, F.; Scotté, M.; Triboulet, J.-P.; Mariette, C.; Chiche, L.; et al. Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: Incidence, prognosis, and risk factors. Am. J. Surg. 2009, 197, 702–709.
- Denbo, J.W.; Orr, W.S.; Zarzaur, B.L.; Behrman, S.W. Toward defining grade C pancreatic fistula following pancreaticoduodenectomy: Incidence, risk factors, management and outcome. HPB 2012, 14, 589–593.
- Luu, A.M.; Krasemann, L.; Fahlbusch, T.; Belyaev, O.; Janot-Matuschek, M.; Uhl, W.; Braumann, C. Facing the surgeon’s nightmare: Incidence and management of postoperative pancreatic fistulas grade C after pancreaticoduodenectomy based on the updated definition of the International Study Group of Pancreatic Surgery (ISGPS). J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 171–181.
- Hirono, S.; Shimokawa, T.; Nagakawa, Y.; Shyr, Y.; Kawai, M.; Matsumoto, I.; Satoi, S.; Yoshitomi, H.; Okabayashi, T.; Motoi, F.; et al. Risk factors for pancreatic fistula grade C after pancreatoduodenectomy: A large prospective, multicenter Japan-Taiwan collaboration study. J. Hepato-Biliary-Pancreat. Sci. 2020, 27, 622–630.
- Kopljar, M.; Čoklo, M.; Krstačić, A.; Krstačić, G.; Jeleč, V.; Zovak, M.; Pavić, R.; Kondža, G. Retrorenal fat predicts grade C pancreatic fistula after pancreaticoduodenectomy. ANZ J. Surg. 2020, 90, 2472–2477.
- Chiba, N.; Ochiai, S.; Yokozuka, K.; Gunji, T.; Sano, T.; Tomita, K.; Tsutsui, R.; Kawachi, S. Risk Factors for Life-threatening Grade C Postoperative Pancreatic Fistula After Pancreatoduodenectomy Compared to Grade B. Anticancer Res. 2019, 39, 2199–2205.
- Lee, H.-J.; Kim, J.W.; Hur, Y.H.; Lee, B.K.; Cho, S.B.; Hwang, E.C.; Lee, S.J.; Yoon, E.J.; Seon, H.J. Multidetector CT findings differ between surgical grades of pancreatic fistula after pancreaticoduodenectomy. Eur. Radiol. 2019, 29, 2399–2407.
- Yang, J.; Li, Y.-C.; Liu, X.-B.; Tan, C.-L. Infection and image findings to predict delayed hemorrhage in postoperative pancreatic fistula patients after pancreaticoduodenectomy. Asian J. Surg. 2022, 45, 1130–1131.
- Keck, T.; Wellner, U.F.; Bahra, M.; Klein, F.; Sick, O.; Niedergethmann, M.; Wilhelm, T.J.; Farkas, S.A.; Börner, T.; Bruns, C.; et al. Pancreatogastrostomy versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann. Surg. 2016, 263, 440–449.
- Perivoliotis, K.; Sioka, E.; Tatsioni, A.; Stefanidis, I.; Zintzaras, E.; Zacharoulis, D. Pancreatogastrostomy versus Pancreatojejunostomy: An Up-to-Date Meta-Analysis of RCTs. Int. J. Surg. Oncol. 2017, 2017, 7526494.
- Cheng, Y.; Briarava, M.; Lai, M.; Wang, X.; Tu, B.; Cheng, N.; Gong, J.; Yuan, Y.; Pilati, P.; Mocellin, S. Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst. Rev. 2017, 9, CD012257.
- Ratnayake, C.B.; Wells, C.I.; Kamarajah, S.K.; Loveday, B.; Sen, G.; French, J.J.; White, S.; Pandanaboyana, S. Critical appraisal of the techniques of pancreatic anastomosis following pancreaticoduodenectomy: A network meta-analysis. Int. J. Surg. 2020, 73, 72–77.
- Jin, Y.; Feng, Y.-Y.; Qi, X.-G.; Hao, G.; Yu, Y.-Q.; Li, J.-T.; Peng, S.-Y. Pancreatogastrostomy vs pancreatojejunostomy after pancreaticoduodenectomy: An updated meta-analysis of RCTs and our experience. World J. Gastrointest. Surg. 2019, 11, 322–332.
- Wang, W.; Zhang, Z.; Gu, C.; Liu, Q.; Liang, Z.; He, W.; Chen, J.; Lai, J. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: A network meta-analysis of randomized control trials. Int. J. Surg. 2018, 57, 111–116.
- Lyu, Y.; Li, T.; Cheng, Y.; Wang, B.; Chen, L.; Zhao, S. Pancreaticojejunostomy Versus Pancreaticogastrostomy After Pancreaticoduodenectomy: An Up-to-date Meta-analysis of RCTs Applying the ISGPS (2016) Criteria. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 139–146.
- Wang, X.X.; Yan, Y.K.; Dong, B.L.; Li, Y.; Yang, X.J. Pancreatic outflow tract reconstruction after pancreaticoduodenectomy: A meta-analysis of randomized controlled trials. World J. Surg. Oncol. 2021, 19, 203.
- Andrianello, S.; Marchegiani, G.; Malleo, G.; Masini, G.; Balduzzi, A.; Paiella, S.; Esposito, A.; Landoni, L.; Casetti, L.; Tuveri, M.; et al. Pancreaticojejunostomy with Externalized Stent vs Pancreaticogastrostomy with Externalized Stent for Patients with High-Risk Pancreatic Anastomosis: A Single-Center, Phase 3, Randomized Clinical Trial. JAMA Surg. 2020, 155, 313–321.
- Senda, Y.; Shimizu, Y.; Natsume, S.; Ito, S.; Komori, K.; Abe, T.; Matsuo, K.; Sano, T. Randomized clinical trial of duct-to-mucosa versus invagination pancreaticojejunostomy after pancreatoduodenectomy. Br. J. Surg. 2018, 105, 48–57.
- Lyu, Y.; Li, T.; Wang, B.; Cheng, Y.; Zhao, S. Selection of pancreaticojejunostomy technique after pancreaticoduodenectomy: Duct-to-mucosa anastomosis is not better than invagination anastomosis: A meta-analysis. Medicine 2018, 97, e12621.
- Cao, Z.; Luo, W.; Qiu, J.; Liu, Y.; Zheng, L.; Zhang, T. Is Invagination Anastomosis More Effective in Reducing Clinically Relevant Pancreatic Fistula for Soft Pancreas after Pancreaticoduodenectomy Under Novel Fistula Criteria: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 1637.
- Kleespies, A.; Rentsch, M.; Seeliger, H.; Albertsmeier, M.; Jauch, K.; Bruns, C.J. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br. J. Surg. 2009, 96, 741–750.
- Casadei, R.; Ricci, C.; Ingaldi, C.; Alberici, L.; De Raffele, E.; Minni, F. Comparison of Blumgart Anastomosis with Duct-to-Mucosa Anastomosis and Invagination Pancreaticojejunostomy after Pancreaticoduodenectomy: A Single-Center Propensity Score Matching Analysis. J. Gastrointest. Surg. 2021, 25, 411–420.
- Menonna, F.; Napoli, N.; Kauffmann, E.F.; Iacopi, S.; Gianfaldoni, C.; Martinelli, C.; Amorese, G.; Vistoli, F.; Boggi, U. Additional modifications to the Blumgart pancreaticojejunostomy: Results of a propensity score-matched analysis versus Cattel-Warren pancreaticojejunostomy. Surgery 2021, 169, 954–962.
- Ke, F.-Y.; Wu, X.-S.; Zhang, Y.; Zhang, H.-C.; Weng, M.-Z.; Liu, Y.-B.; Wolfgang, C.; Gong, W. Comparison of postoperative complications between internal and external pancreatic duct stenting during pancreaticoduodenectomy: A meta-analysis. Chin. J. Cancer Res. 2015, 27, 397–407.
- Hong, S.; Wang, H.; Yang, S.; Yang, K. External stent versus no stent for pancreaticojejunostomy: A meta-analysis of randomized controlled trials. J. Gastrointest. Surg. 2013, 17, 1516–1525.
- Patel, K.; Teta, A.; Sukharamwala, P.; Thoens, J.; Szuchmacher, M.; DeVito, P. External pancreatic duct stent reduces pancreatic fistula: A meta-analysis and systematic review. Int. J. Surg. 2014, 12, 827–832.
- Pessaux, P.; Sauvanet, A.; Mariette, C.; Paye, F.; Muscari, F.; Cunha, A.S.; Sastre, B.; Arnaud, J.-P. External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: Prospective multicenter randomized trial. Ann. Surg. 2011, 253, 879–885.
- Wang, G.; Li, L.; Ma, Y.; Qu, F.-Z.; Zhu, H.; Lv, J.-C.; Jia, Y.-H.; Wu, L.-F.; Sun, B. External Versus Internal Pancreatic Duct Drainage for the Early Efficacy after Pancreaticoduodenectomy: A Retrospectively Comparative Study. J. Investig. Surg. 2016, 29, 226–233.
- Jiang, Y.; Chen, Q.; Shao, Y.; Gao, Z.; Jin, M.; Gao, B.; Zhou, B.; Yan, S. The prognostic value of external vs internal pancreatic duct stents after pancreaticoduodenectomy in patients with FRS ≥ 4: A retrospective cohort study. BMC Surg. 2021, 21, 81.
- Stoop, T.F.; Ghorbani, P.; Scholten, L.; Bergquist, E.; Ateeb, Z.; van Dieren, S.; Holmberg, M.; Besselink, M.G.; Sparrelid, E.; Del Chiaro, M. Total pancreatectomy as an alternative to high-risk pancreatojejunostomy after pancreatoduodenectomy: A propensity score analysis on surgical outcome and quality of life. HPB 2022, 24, 1261–1270.
- Marchegiani, G.; Perri, G.; Burelli, A.; Zoccatelli, F.; Andrianello, S.; Luchini, C.; Donadello, K.; Bassi, C.; Salvia, R. High-risk Pancreatic Anastomosis versus Total Pancreatectomy after Pancreatoduodenectomy: Postoperative Outcomes and Quality of Life Analysis. Ann. Surg. 2022, 276, e905–e913.
- Salvia, R.; Lionetto, G.; Perri, G.; Malleo, G.; Marchegiani, G. Total pancreatectomy and pancreatic fistula: Friend or foe? Updates Surg. 2021, 73, 1231–1236.
- Aoki, T.; Sakamoto, Y.; Kohno, Y.; Akamatsu, N.; Kaneko, J.; Sugawara, Y.; Hasegawa, K.; Makuuchi, M.; Kokudo, N. Hepatopancreaticoduodenectomy for Biliary Cancer: Strategies for Near-zero Operative Mortality and Acceptable Long-term Outcome. Ann. Surg. 2018, 267, 332–337.
- Hasegawa, K.; Kokudo, N.; Sano, K.; Seyama, Y.; Aoki, T.; Ikeda, M.; Hashimoto, T.; Beck, Y.; Imamura, H.; Sugawara, Y.; et al. Two-stage pancreatojejunostomy in pancreaticoduodenectomy: A retrospective analysis of short-term results. Am. J. Surg. 2008, 196, 3–10.
- Yamazaki, S.; Takayama, T.; Mitsuka, Y.; Yoshida, N.; Shimamoto, N.; Higaki, T. Feasibility of Hyaluronate Carboxymethylcellulose-Based Bioresorbable Membrane in Two-Staged Pancreatojejunostomy. World J. Surg. 2020, 44, 902–909.
- Zhou, Y.-M.; Zhou, X.; Wan, T.; Xu, D.; Si, X.-Y. An evidence-based approach to the surgical interventions for severe pancreatic fistula after pancreatoduodenectomy. Surgeon 2018, 16, 119–124.
- Wroński, M.; Cebulski, W.; Witkowski, B.; Guzel, T.; Karkocha, D.; Lech, G.; Słodkowski, M. Surgical management of the grade C pancreatic fistula after pancreatoduodenectomy. HPB 2019, 21, 1166–1174.
- Záruba, P.; Rousek, M.; Kočišová, T.; Havlová, K.; Ryska, M.; Pohnán, R. A comparison of surgical approaches in the treatment of grade C postoperative pancreatic fistula: A retrospective study. Front. Surg. 2022, 9, 927737.
- Groen, J.V.; Smits, F.J.; Koole, D.; Besselink, M.G.; Busch, O.R.; Dulk, M.D.; van Eijck, C.H.J.; Koerkamp, B.G.; van der Harst, E.; de Hingh, I.H.; et al. Completion pancreatectomy or a pancreas-preserving procedure during relaparotomy for pancreatic fistula after pancreatoduodenectomy: A multicentre cohort study and meta-analysis. Br. J. Surg. 2021, 108, 1371–1379.
- Paye, F.; Lupinacci, R.M.; Kraemer, A.; Lescot, T.; Chafaï, N.; Tiret, E.; Balladur, P. Surgical treatment of severe pancreatic fistula after pancreaticoduodenectomy by wirsungostomy and repeat pancreatico-jejunal anastomosis. Am. J. Surg. 2013, 206, 194–201.
- Ribero, D.; Amisano, M.; Zimmitti, G.; Giraldi, F.; Ferrero, A.; Capussotti, L. External Tube Pancreatostomy Reduces the Risk of Mortality Associated with Completion Pancreatectomy for Symptomatic Fistulas Complicating Pancreaticoduodenectomy. J. Gastrointest. Surg. 2013, 17, 332–338.
- Xu, J.; Dai, X.; Bu, X.; Gao, F.; Zhang, X. Pancreaticojejunal Bridge-Anastomosis: A Novel Option for Surgeon to Preserve Pancreatic Body and Tail in Urgent Reoperation for Intra-abdominal Massive Hemorrhage after Pancreaticoduodenectomy. World J. Surg. 2010, 34, 2457–2462.
- Kent, T.S.; Callery, M.P.; Vollmer, C.M., Jr. The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB 2010, 12, 577–582.
