This literature review aims to analyze all the vaccine candidates that went to phase II and above with the rationale behind the completion of some vaccines to phase III and why others failed to continue to phase III. Moreover, this paper will provide comprehensive information about COVID-19 infection, its symptoms, the structure of the virus, the variants that we have become aware off and how they differ. Furthermore, it will inform the literature about vaccines that developed for COVID-19 infection, vaccine vehicles and trials on vaccines. This paper shall open doors for further research and better understanding for reasons that hinder vaccines from reaching the market, which is important to be taken into consideration when developing new vaccines for viruses in the future.
- severe acute respiratory syndrome coronavirus 2
- SAR-CoV-2
- pandemic
- vaccines
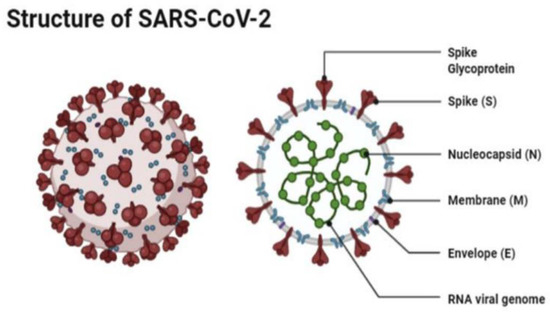
COVID-19 Variants
Viruses innately have the ability to mutate constantly and lead to variants. Some variants emerge and disappear while some persist (3). Mutations to the virus happens during the process of viral replication, that is when the virus attaches to the ACE2 receptor, which is present on the membrane of the host cell. In viral replication and amplification, the assembly of the virions is carried out in the host cell endoplasmic reticulum and Golgi apparatus. During this process errors can occur in the genome leading to mutations which give rise to variants (3). A change in the genetic sequence is called a mutation. Mutations can increase the transmissibility and/or virulence of the virus with a possible reduction of vaccine effectiveness (13). Genomes that differ from each other in genetic sequence are called variants. Variants can differ from each other by one or more mutations. When a phenotypic difference is demonstrated among the variants, they are called strains (3). So far, Covid-19 has been defined by 17 known mutations (14 non-synonymous mutations and 3 deletions), eight of these mutations have been on the spike protein, the main target site for the vaccination, with at least three of these mutations having a significant biological effect (14). Several human Coronaviruses (alpha-CoVs, HCoVs-NL63, beta-CoVs, HCoVs-OC43, HCoVs-229E, HCoVs-HKU1, MERS-CoV, SARS-CoV and ARDS) have been identified (14). New versions of the Coronavirus Vaccines will appear due to the large genomic potential, rapid mutation capabilities and high prevalence. The US Department of Health and Human Services (HHS) established the SARS-CoV-2 Interagency Group (SIG) to focus on the rapid characterization of emerging variants and actively monitor their potential impact on critical SARS-CoV-2 countermeasures, such as immunizations, pharmaceuticals, and testing. SARS-CoV-2 variations are divided into four categories under the SIG Variant classification scheme: variants being monitored (VBM), variants of interest (VOI), variants of concern (VOC), and variants of high consequence (VOHC) (15).The following are some of the variants of coronavirus:
- 20A/S:439K
This variant The 20A/S:439K variant was initially found in Ireland (16). It has S:N439K mutation with the deletions of amino acids at positions 69 and 70 of S proteins that results in an increase in ACE2 binding, resistance to antibodies and convalescent plasma (17).
- 20A/S:98F
The 20A/S:98F variant has S:98F mutation which was found predominantly in Belgium and Netherlands (17).
- 20C/S:80Y
The 20C/S:80Y variant had 18 nucleotide mutations, possibly related to apolipoprotein B editing complex (APOBEC)-like editing within the host which are found in at least 10 countries in Europe (17).
- 20B/S:626S
The 20B/S:626S variant has S:626S mutation. This variant is found in 15 countries of Europe that is predominantly seen in Norway, Denmark, and the UK (17).
- 20B/S:1122L
The 20B/S:1122L variant has S:V1122L mutation and is found predominantly in Sweden, Norway, and Denmark (17).
- N440K
Another new variant N440K resulted in the sudden increase in cases in Andhra Pradesh, India. N440K is a new variant with the mutation in the S protein, which has enhanced binding to ACE2 receptors, 10 to 1,000 folds more transmissible and resistant to class 3 monoclonal antibodies C135 and REGN10987 (17).
- 20A.EU1/ S:A222V
The 20A.EU1 variant has non-terminal domain (NTD) mutations which do not play a direct role in receptor binding or membrane fusion. This variant was initially identified on 20 June, 2020 in Spain but rapidly spread across Europe and many countries (17).
- 20A.EU2
The 20A.EU2 variant was found in France in June 2020 and has become the second dominant variant in Europe. The notable mutations are S477N, E484K, and N501Y, which demonstrated slight increase in ACE2 binding, resistance to multiple antibodies and convalescent sera (17).
- B.1.526 (20C/S:484K) and B.1.525 (20A/S:484K)
These variants were first identified in New York, USA. The notable mutations are E484K and S477N. While E484K decreases antibody response, S477N increases the attachment process (17).
- Double mutant variant (B.1.617)
This variant is first detected in India. As two mutations are seen in the same virus, this variant is called a “double mutant” variant. There was a significant increase in COVID-19 cases in India. The notable mutations are E484Q and L452R. These variants are at increased risk of transmission and also resistant to vaccination (17).
- Triple mutant variant (B.1.618)
In addition to E484Q and L425R in double mutant variants, the new triple variant discovered on April 20 2021, is characterized by the deletion of two amino acids, H146del and Y145del in the S protein (17).
- US Southern California variant (CAL.20C)
Vaccines:
With the economic, societal and public health effects of COVID-19, it was essential to develop vaccine to minimize the severe consequences of this virus. Before the development of the vaccines, some non- pharmaceutical interventions have shown benefits in minimizing the spread of COVID-19. Those non-pharmaceutical interventions included social distancing, wearing of the facemasks and limits of large gatherings. However, they had limited effectiveness due to poor adherence with those practices and unclear advices from ministries of public health. Therefore, the development of an effective COVID-19 vaccine has been a critical need to control the disease and its effects (18).
Global collaboration among pharmaceutical companies, governments and academic researchers were mounted to develop COVID-19 vaccine since a publication about the SARS-CoV-2 viral sequence was released on January 10, 2020. The three main authorities that coordinated the vaccine research were World Health Organization, Gavi and coalition for Epidemic Preparedness and Innovation (CEPI). It was reported by CEPI that there were 321 vaccine candidates in development around the globe in September 2020. However, only 40 vaccine candidates have progressed to clinical trials in humans in October 2020 and 11 of them were in phase 3 clinical trials that aims to provide the safety and efficacy evidence that is required for approval of the vaccine.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10122086
