Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Gastroenterology & Hepatology
Hepatitis C is a liver infection caused by the hepatitis C virus and is a major health problem that contributes to the global burden of chronic disease. Chronic infection can lead to liver cancer and death from end-organ damage. Despite the introduction of novel anti-viral therapy, the disease burden is still high.
- extrahepatic manifestation
- hepatitis C virus
- hepatitis C infection
1. Introduction
Over 58 million people worldwide are infected with the hepatitis C virus (HCV), an estimated 2.4 million people in the United States live with hepatitis C, and about 400,000 people died from this disease in 2016 [1,2]. Chronic hepatitis C is associated with significant morbidity, and although the number of cases is decreasing, it is still a common reason for liver transplantation in the United States [3].
Approximately 25% of HCV-infected patients spontaneously clear the infection [4], but most patients become chronically infected with HCV and develop liver-related complications, including decompensated cirrhosis and hepatocellular carcinoma (HCC), which significantly contribute to mortality. However, non-liver-related hepatitis C manifestations can also develop in chronically hepatitis C-infected patients, and these extrahepatic manifestations also contribute to the disease burden, poor outcomes, and mortality in HCV-infected patients [5,6,7].
Extrahepatic manifestations of HCV can involve almost every organ system in the human body and include metabolic syndromes (diabetes mellitus, cardiovascular disease, cerebrovascular disease), autoimmune diseases (Sjogren syndrome, thyroiditis, arthritis), immune-mediated disorders (mixed cryoglobulinemia), malignancy (lymphoma), dermatologic conditions (lichen planus, porphyria cutanea tarda), and renal diseases [8,9,10] (Figure 1). These extrahepatic manifestations of HCV can increase mortality in chronic hepatitis C-infected patients and increase the risk of developing hepatic fibrosis and HCC; they also reduce the quality of life in patients and increase health care costs worldwide.
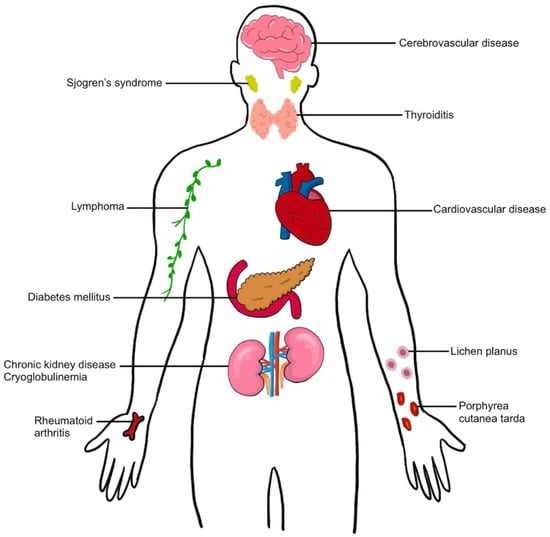
Figure 1. Extrahepatic manifestation in hepatitis C virus infection.
The primary event in hepatitis C infections involves viral replication in hepatocytes. Chang and co-investigators measured HCV replication in the human liver using in situ hybridization techniques to measure the HCV genome and replicative intermediate ribonucleic acids (RNAs) [11]. They determined that the number of HCV genomes ranged from 7-64 RNA molecules in individual hepatocytes. The maximum number of RNA genomes for a single cell was 74, and the number in the entire liver ranged from 1.8 × 1011 molecules to 1.8 × 1012 molecules. There was a gradient of dispersion around infected hepatocytes which suggested that infection spread to neighboring hepatocytes as the mechanism of viral spread in the liver. In addition, viral synthetic activities can compromise the normal metabolic activities in hepatocytes and increase the possibility of hepatocellular injury and death. The number of genomes per milliliter (mL) serum in the Chang study ranged from 3.4 × 106 molecules to 5.0 × 108 molecules. Schijman et al. determined the HCV load in 245 male and female patients with HCV infection. The median HCV load was 344,000 international units/mL [12]. There were no major differences between male and female patients or between different viral genotypes (1a, 1b, 2, 3a, 4). These virions in the serum have the potential to reinfect hepatocytes and infect extrahepatic tissues.
The development of extrahepatic complications associated with hepatitis C infection involves complex interactions which include direct viral effects on tissue, the metabolic effects associated with hepatic infection and injury, and the host defense responses associated with ongoing infection [13]. Other factors which potentially influence the development of extrahepatic manifestations include obesity, alcohol use, and the viral genotype causing the infection. Metabolic consequences will also depend on the duration of the infection, the possibility of co-infection with other viral pathogens, and drug treatment effects. These various possibilities are discussed below in the sections on non-hepatic organ involvement in hepatitis C infections.
With new HCV treatments based on pangenotypic direct-acting antiviral (DAAs) therapy, over 90% of hepatitis C infected patients can have sustained virologic responses (SVR) within 2–3 months, and these regimens can be used in many patients with comorbidities who previously could not be treated [14]. Further, recent studies show that SVR was associated with a significant reduction in the risk of several extrahepatic manifestations of HCV [15]. HCV treatment can reduce medical costs by up to $25,000 per patient per year [16]. Therefore, the purpose of this review is to analyze the risk factors, disease burden, outcomes, and comorbidities of each extrahepatic manifestation of HCV to identify possible research priorities for future investigation. Despite the introduction of DAAs and the more than 90% rate of SVR, about 38% of patients with chronic HCV infection develop at least one extrahepatic manifestation [17] (Table 1 and Table 2).
Table 1. Prevalence of extrahepatic manifestations in HCV infections.
| EHMs | Authors | Study Method | Findings in HCV Patients (95%CI) |
|---|---|---|---|
| Diabetesmellitus | Younossi [9] | Systematic review (31 studies, n = 263,973) |
Prevalence: 15% (13–18%) |
| Younossi [18] | Systematic review (21 studies, n = 22,432) |
Prevalence 19.0% (15.6–22.9%) | |
| Cardiovascular and cerebrovascular disease | Lee [19] | Systematic review (36 studies, n = 341,739) |
RR of cardiovascular events, MI, stroke 1.28 (1.15–1.42), 1.13 (1.00–1.28), 1.28 (1.18–1.39), respectively |
| Petta [20] | Systematic review (22 studies, n = 390 602) |
OR of CVD–related mortality, carotid plaques, and CVA 1.65 (1.07–2.56), 2.27(1.76–2.94), 1.30 (1.10–1.55), respectively |
|
| Mixed cryoglobulinemia | Younossi [9] | Systematic review (21 studies, n = 4415) |
Prevalence: 30% (21.4–38.9%) OR 11.50 (4.56–29.00) |
| Park [21] | Retrospective cohort (n = 55,646) | HR 16.91 (12.00–23.81) | |
| Chronic kidney disease | Park [21] | Retrospective cohort (n = 56,448) |
HR of 1.27 (1.18–1.37) |
| Lymphoma | de Sanjose [22] | Case control (n = 11,053) |
OR of Marginal zone lymphoma, DLBCL, and lymphoplasmacytic lymphoma 2.47 (1.44–4.23), 2.24 (1.68–2.99), 2.57 (1.14–5.79), respectively |
| Pozzato [23] | Systematic review (50 studies, n = 21,262) |
RR of NHL 2.3 (1.8–2.9) | |
| Porphyria cutanea tarda | Gisbert [24] | Systematic review (50 studies, n = 2167) |
Prevalence: 47–50% OR 275 (104–725) |
| Younossi [9] | Systematic review (7 studies, n = 970,315) |
Prevalence: 0.5% (0.1–0.8) OR 8.53 (4.15–17.52) |
|
| Lichen planus | Alaizari [25] | Systematic review (19 studies, n = 4326) |
OR 6.07 (2.73–13.48) |
| Sjogren syndrome | Younossi [9] | Systematic review (11 studies, n = 38,789) |
Prevalence: 11.9% (7.6–16.2%) RR 2.29 (0.19–27.09) |
| Yeh [26] | A population-based analysis (n = 48,145) |
OR 2.49 (2.16–2.86) | |
| Rheumatoid arthritis | Younossi [9] | Systematic review (4 studies, n = 210,538) |
Prevalence: 1% (0.0–2.0%) OR 2.39 (1.52–3.77) |
| Younossi [18] | Systematic review (5 studies, n = 18,234) |
Prevalence: 4.5% (0.6–25.7%) OR 2.49 (1.79–3.45) |
|
| Thyroiditis | Shen [27] | Systematic review (12 studies, n = 3603) |
Prevalence of hypothyroidism: 6.36% OR 3.10 (2.19–4.40) |
EHMs, extrahepatic manifestations; OR, odd ratio; RR, relative risk; HR, hazard ratio; MI, myocardial ischemia; CVD, cardiovascular disease; CVA, cerebrovascular accident; DLBCL, diffuse large B cell lymphoma; NHL, Non-Hodgkin’s lymphoma.
Table 2. Independent factors and disease burdens of EHMs.
| EHM | Independent Factor | Disease Burden |
|---|---|---|
| Diabetes mellitus [28,29,30,31] |
Cirrhosis, aging, obesity, family history of DM, HCV genotype (1,2,4) | Increased risk of hepatic fibrosis, Increased risk of HCC |
| Cardiovascular disease [19,20,32,33,34] |
DM, HTN, HIV coinfection | Increased risk of MI, cardiac dysfunction, heart failure |
| Mixed cryoglobulinemia and renal disease [21,35] | Cardiovascular disease, liver failure, infections, chronic renal failure | Increased risk of CKD |
| Lymphoma [36,37] |
Geographic variations | Increased risk of developing chronic hepatitis, cirrhosis, and HCC |
| Sjogren syndrome [38,39,40] |
Older age, liver disease activity | May increase risk of developing MALT lymphoma, malignant B cell non-Hodgkin lymphoma |
| Rheumatoid arthritis [41] |
Smoking, previous history of arthritis | Data limited |
| Thyroiditis [27,42,43] |
Female, geographic variability | Data limited |
DM, Diabetes mellitus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HTN, hypertension; HIV, human immunodeficiency virus; MI, myocardial ischemia; CKD, chronic kidney disease.
2. Skin Manifestations in Hepatitis C Virus Infection
A variety of skin diseases are associated with HCV infection; these include mixed cryoglobulinemia, porphyria cutanea tarda (PCT), and lichen planus. Cutaneous manifestations are present in up to 17% of patients with HCV infection [125].
2.1. Porphyria Cutanea Tarda
Porphyria cutanea tarda is a dermatological complication that presents with blistering skin lesions forming vesicles or bullae on sun-exposed areas. It is caused by excess uroporphyrin in the skin due to the deficiency of uroporphyrinogen decarboxylase activity, an hepatic enzyme [126,127].
2.1.1. Risk Factors & Prevalence
Multiple studies have demonstrated a strong association between PCT and HCV infection. A systematic review conducted by Gisbert and colleagues analyzed 50 studies and reported the prevalence of HCV in PCT patients was 47% (OR 275, 95% CI 104–725) [24]. Similarly, Younossi and colleagues reported an increased risk of developing PCT in HCV patients (OR 8.53, 95% CI 4.15–17.52) [9]. The prevalence of anti-HCV in PCT varies significantly with geographic distribution; a higher prevalence is found in Southern Europe, and a lower prevalence is found in Northern Europe [128].
2.1.2. Mechanism
The mechanism through which HCV infection may cause or trigger PCT is unknown. One possible pathway involves iron overload and oxidative stress [129]. Chronic infection of HCV can lead to progressive iron accumulation [130]. One study reported that patients with HCV infections with PCT have a higher accumulation of iron in the liver compared to patients with HCV without PCT [130]. Increased hepatic iron has a central role in the pathogenesis of PCT, and factors that increase susceptibility include alcohol, smoking, iron overload, estrogen therapy, homeostatic iron regulator (HFE) mutation, and HCV infection [129,131,132]. Sastre demonstrated that urine porphyrin can be detected in patients with HCV infections who do not have clinical symptoms of PCT and reported the clearance of urine porphyrin after SVR following treatment with DAAs [133].
2.1.3. Burden and Outcome after Treatment
Studies have shown IFN-α treatment may precipitate PCT relapse [134]. Previous studies recommended reducing iron overload by phlebotomy before initiating IFN-based therapies, which produced a better response and improved SVR rates in chronic HCV infection [135]. With DAAs therapy, porphyrin levels are decreased significantly or completely reduced to normal levels, but data are limited [134]. A recent study by García-Fraile recruited 13 patients with HCV infection, and PCT demonstrated that SVR after DAAs treatment leads to PCT resolution [136].
2.2. Lichen Planus
Lichen planus is a chronic inflammatory disorder affecting the skin and mucosal surfaces, it is a T-cell mediated disease affecting stratified squamous epithelium of the skin and/or mucus membranes. The classic manifestations include pruritic, polygonal, and purple papules or plaques, and the condition commonly affects middle-aged adults. Lichen planus may appear in the skin, mucous membranes, scalp, nails, and genitalia. Oral lichen planus presents with multiple, symmetrical lesions that can have a reticular, plaque-like, papular, atrophic, erosive, or vesicular-bullous forms in the oral mucus membrane [137].
2.2.1. Risk Factor and Prevalence
The association between HCV and lichen planus remains controversial [138]. A systematic review included 6378 HCV patients with lichen planus and reported the prevalence of HCV infection in lichen planus was 22.3% [139]. Based on a meta-analysis, HCV seropositivity was more prevalent in oral lichen planus patients than controls (OR 6.07, 95% CI 2.73–13.48) [25]. However, a study conducted in central Germany showed no association between lichen planus and HCV [140]. Genetic variability may influence the risk of disease presentation [141].
2.2.2. Mechanism
The exact etiology of lichen planus in chronic HCV infection is still unknown; the pathogenesis may involve an immunological process in which cytotoxic CD8+ T cells cause apoptosis of the basal cells of the oral epithelium [142]. A direct cytopathic effect from HCV in the development of lichen planus is possible [143]. Hepatitis C virus RNA has been found in biopsy specimens. Another hypothesis suggests that circulating autoantibodies in HCV patients promote B-cell proliferation and that host immune response with the production of proinflammatory cytokines as a response to HCV causes skin disease [143]. Genetic factors have been considered a possible factor in the development of oral lichen planus in HCV-infected patients involving HLA-DR6 compared to those without HCV infection. However, this study was conducted in Italy, and geographic differences have been postulated as a factor in developing oral lichen planus [144,145]. Another study showed that patients with oral lichen planus and HCV infection have higher levels of CD8+ lymphocytes in lamina propria compared with patients with oral lichenoid reaction [146]. Figueiredo hypothesized that the host immune system is responsible for oral lichen planus more than direct viral effects [147].
2.2.3. Burden and Outcomes after Treatment
Interferon (IFN) therapy is controversial in the management of HCV in patients with comorbid lichen planus, as there have been reports of both improvement and aggravation of lichen planus symptoms [148,149,150]. Studies on treatment with IFN-free DAAs are limited, and a case series with a small sample reported successful outcomes in HCV-associated oral lichen planus in all seven patients [151].
This entry is adapted from the peer-reviewed paper 10.3390/biology12010023
This entry is offline, you can click here to edit this entry!
