1. Introduction
Reactive oxygen species (ROS) are a series of molecular oxygen derivatives that regulate numerous physiological and pathological processes [
1]. As a group of chemical species, ROS can be categorized into non-radical and free radical species. Non-radical ROS include hydrogen peroxide (H
2O
2), organic hydroperoxides (ROOH), singlet molecular oxygen (
1O
2), ozone (O
3), hypochlorous acid (HOCl), and hypobromous acid (HOBr), whereas free radical ROS include superoxide anion radical (O
2•−), hydroxyl radical (·OH), peroxyl radical (ROO·) and alkoxyl radical (RO·) [
2]. Cellular ROS are generated endogenously through mitochondrial oxidative phosphorylation, or exogenously stimulated by xenobiotics, cytokines, and bacterial infection [
3,
4]. O
2•− and H
2O
2 are acknowledged as the most physiologically relevant ROS. O
2•− is mainly generated by nicotinamide adenine dinucleotide phosphate oxidases (NOXs), or the complexes I and III of the mitochondrial electron transport chain (ETC). H
2O
2 is generated by superoxide dismutases (SOD), NOX4, monoamine oxidases, and xanthine oxidases within a few different organelles (endoplasmic reticulum, mitochondria, and peroxisomes). More importantly, ROS are required for numerous cellular processes including cell growth, differentiation, and death by acting as signalling molecules [
5]. Multiple signalling pathways are involved in mediating ROS, including nuclear factor kappa-B (NF-κB), mitogen-activated protein kinase (MAPK) cascade, Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2-antioxidant response elements (Keap1-Nrf2-ARE) signalling, adenosine 5′-monophosphate-activated protein kinase (AMPK), phosphoinositide-3 kinase-(PI3K-) Akt pathway, etc. [
6,
7].
The dynamic balance between ROS production and antioxidant capacity responsibly maintains the cellular redox homeostasis [
8]. However, oxidative stress occurs when elevated ROS overwhelms the cellular antioxidant defence, damaging nucleic acids, proteins, and lipids [
4,
5,
9]. To protect cells from oxidative damage as well as to maintain physiological ROS levels, organisms have developed antioxidant defence systems that comprise small-molecular-weight antioxidants and antioxidant enzymes. Small-molecular-weight antioxidants include glutathione (GSH), cysteine, ascorbic acid, and α-tocopherol, while antioxidant enzymes include SOD, catalase (CAT), glutathione peroxidase (GPX), glutaredoxin (GRX), thioredoxin (TXN), and peroxiredoxin (PRX) [
10,
11,
12]. From a pharmacokinetic point of view in scavenging intracellular ROS, targeting antioxidant enzymes is more effective than small-molecule antioxidants, and ultimately maintains the antioxidant defence [
13]. A series of antioxidant enzymes such as CAT, GPX, and PRX eliminate H
2O
2 by converting H
2O
2 into H
2O [
14,
15,
16]. On the other hand, SOD is the most powerful O
2•− scavenger, which catalyses the dismutation of O
2•− into H
2O
2 and O
2 [
17]. Currently, three isoforms of SOD have been discovered in mammalian cells, namely, copper–zinc superoxide dismutase (Cu/ZnSOD, SOD1), manganese superoxide dismutase (MnSOD, SOD2), and the extracellular superoxide dismutase (EcSOD, SOD3) [
5]. Of these isoforms, MnSOD garners widespread interest as it potently scavenges mitochondrial O
2•−, which is the major source of cellular ROS [
18]. The indispensable role of MnSOD is further highlighted by the evidence of significant neonatal mortality in MnSOD-deficient mice, compared to the considerable neonatal survival in the SOD1- or SOD3-deficient mice [
19,
20,
21,
22].
MnSOD is a nuclear-encoded enzyme that translocates into the mitochondrial matrix [
12]. It is constituted by a homotetramer containing an active site with manganese as a cofactor. Despite its enzymatic antioxidant function, MnSOD readily binds with iron, and the produced iron-substituted enzyme attains peroxidase activity that generates highly reactive hydroxyl radicals [
23,
24]. Therefore, in protecting against oxidative stress, manganese seems like the perfect metal, while the quaternary structure of MnSOD responsibly maintains the catalytic and dismutase activity [
25]. The expression and activity of MnSOD is regulatable at multiple levels, from transcription and translation to posttranslational modifications [
26]. NF-κB, specificity protein 1 (Sp1), activating protein-1 (Ap1), p53, and CCAAT binding protein (C/EBP) are the major transcription factors that regulate MnSOD gene expression by directly binding to specific DNA elements or interacting with its partners [
27,
28]. Furthermore, posttranslational modifications regulate MnSOD protein expression and activity through nitration, phosphorylation, and acetylation. Peroxynitrite (ONOO
-) inactivates MnSOD by nitrating the tyrosine-34 amino acid residue of MnSOD protein. Acetylation at lysine-68, lysine-122, lysine-53, and lysine-89 amino acid residues, also inactivate MnSOD. In addition, sirtuin 3 (SIRT3) reactivates MnSOD by deacetylating the above lysine residues in response to irradiation, nutritional deprivation, and oxidative stress [
25,
27]. Moreover, MnSOD activity and stability are enhanced through phosphorylation at Ser106 by the mitochondrial cell-cycle kinase 1 (Cdk1) [
29].
Considering that MnSOD engages in detoxifying ROS through O
2•− dismutation, which possibly becomes a contributing factor or consequence in multiple diseases, it is vital to understand the physiological and pathological role of MnSOD. Recent evidence has revealed the pathological involvement of MnSOD in a number of diseases (
Figure 1). For example, the enzymatic activity or expression of MnSOD is frequently downregulated in numerous diseases, such as diabetes and neurodegenerative diseases [
30,
31,
32,
33], whereas overexpression of MnSOD protects against pro-oxidant insults resulting from inflammatory cytokines, irradiation, hyperoxic injury, and ischaemia/reperfusion [
34,
35,
36,
37,
38,
39,
40]. In addition, selective MnSOD mimetics have therapeutic potential in treating rheumatoid arthritis (RA), ischaemic stroke, and kidney diseases by mimicking the activity of MnSOD [
41,
42,
43,
44]. Nevertheless, a comprehensive summary of MnSOD regulatory roles in a range of diseases is lacking. In this review, we focus on the mechanisms of MnSOD in regulating various diseases that are associated with imbalanced redox status, including inflammation, fibrotic diseases, diabetes, neurodegenerative diseases, vascular diseases, and cancer (
Figure 2). Advances in the therapeutic application of MnSOD activators and MnSOD mimetics are also discussed (
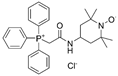
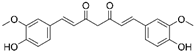
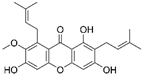
Table 1). Moreover, limitations and critical issues in these MnSOD-related studies are addressed. Further understanding of how MnSOD can be regulated in the clinic may provide strategies and prospects for antioxidant therapy. Additionally, the comprehensive information and scientific advances presented in this review may facilitate the understanding of the physiological and pathological roles of MnSOD.
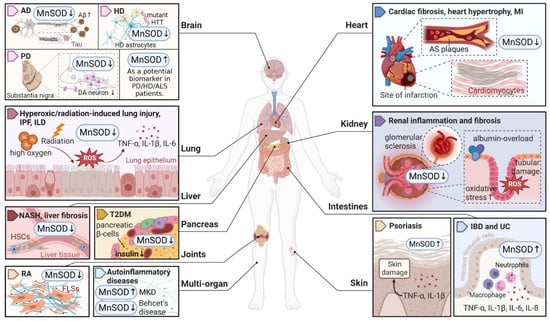
Figure 1. An overview of MnSOD expression in various pathological states of human diseases. Abnormal expression levels of MnSOD have been detected in multiple diseases and have a wide impact on different organs, followed by various pathological alterations such as inflammation, fibrosis, sclerosis, and neuron degeneration. MnSOD, manganese superoxide dismutase; ROS, reactive oxygen species; AD, Alzheimer’s disease; Aβ, amyloid β-protein; HD, Huntington’s disease; HTT, huntingtin; PD, Parkinson’s disease; DA, dopaminergic; ALS, amyotrophic lateral sclerosis; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; TNF, tumour necrosis factor; IL, interleukin; NASH, non-alcoholic steatohepatitis; HSCs, hepatic stellate cells; T2DM, type 2 diabetes mellitus; RA, rheumatoid arthritis; FLSs, fibroblast-like synoviocytes; MKD, mevalonate kinase deficiency; MI, myocardial infarction; AS, atherosclerosis; IBD, inflammatory bowel disease; UC, ulcerative colitis. ‘↑’ represents increased or upregulated, while ‘↓’ represents decreased or downregulated (created with
BioRender.com (accessed on 4 November 2022)).
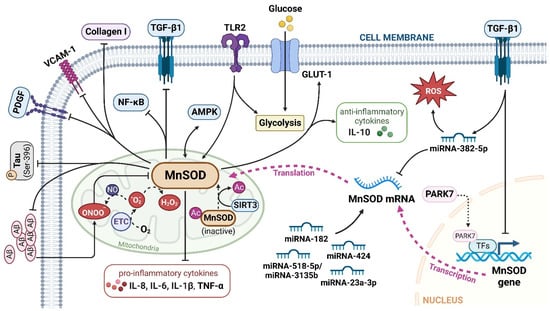
Figure 2. The major signalling pathways involved in MnSOD regulation. MnSOD is localized in the mitochondrial matrix to catalyse the dismutation of O2
•− to H2O2, and regulate cellular redox homeostasis. Multiple factors, cytokines, proteins, and miRNAs have been involved in the transcriptional and translational modulation of MnSOD. TGF-β1, transforming growth factor-β1; TLR2, toll-like receptor 2; Aβ, amyloid, β-protein; GLUT-1, glucose transporter member 1; IL, interleukin; VCAM-1, vascular cell adhesion molecule-1; AMPK, adenosine 5′-monophosphate-activated protein kinase; P, phospho; PDGF, platelet-derived growth factor; NF-κB, nuclear factor kappa-B; ETC, electron transport chain; PARK7, Parkinson disease protein 7; TFs, transcriptional factors; Ac, acetyl; SIRT3, sirtuin 3. ‘↑’ represents increased or upregulated, while ‘⊥’ represents suppressed or downregulated (created with
BioRender.com (accessed on 4 November 2022)).
2.1. Inflammatory Diseases
Inflammation acts as a defensive mechanism to confront harmful stimuli such as infection and tissue damage [
64,
65]. Oxidative stress in activated infiltrating immune cells and tissue-resident immune cells, such as macrophages and T cells, is responsible for inflammation-related diseases [
66,
67]. Numerous studies have demonstrated that MnSOD plays a vital role in protecting organismal integrity and homeostasis by regulating inflammatory chemokines and cytokines [
68,
69] (
Figure 2).
2.2. Fibrotic Diseases
Fibrosis is defined by the excessive accumulation of extracellular matrix (ECM) components such as collagen and fibronectin in damaged tissues, which ultimately induce tissue sclerosis and organ failure [
96]. This process is initiated by the continuous activation and transdifferentiation of local fibroblasts in response to chronic injury [
97]. To date, the regulatory role of MnSOD in the fibrosis of multiple organs has been widely reported and summarized (
Figure 1).
2.3. Diabetes
Diabetes mellitus occurs whereby an abnormally high blood glucose level (hyperglycaemia) results from insulin insufficiency, and/or impaired tissue response to insulin (i.e., insulin resistance) [
119]. It is a heterogeneous disorder with two major forms, type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), both of which are exacerbated by free radicals produced via glucose autoxidation [
120,
121,
122]. Emerging evidence has indicated that the CT genotype of the MnSOD 47C/T gene is a significant risk factor for T1DM susceptibility (OR = 2.37; CI 95% = 1.03 to 5.46;
p = 0.040) [
123]. Here, we summarize the different roles of MnSOD in regulating diabetes and its complications.
2.4. Neurodegenerative Diseases
Neurodegenerative diseases are indicated by progressive degeneration and the selective loss of neuronal systems, leading to cognitive impairment, dementia, motor dysfunction, and even death [
141]. The majority of neurodegenerative diseases are characterized by the accumulation of misfolded proteins in the central nerve system, e.g., amyloid-β (Aβ) and tau aggregates in Alzheimer disease (AD), SOD1 pathology in amyotrophic lateral sclerosis (ALS), and mutated huntingtin (HTT) in Huntington disease (HD), and accompanied by a progressive loss of neurons, e.g., the loss of dopaminergic (DA) neurons in PD (Parkinson’s disease) in the affected regions [
142]. A number of studies have highlighted the potential therapeutic role of MnSOD in detoxifying cerebral ROS to inhibit the aggregation of misfolded proteins and protect against neurodegenerative disorders (
Figure 1).
2.5. Vascular Diseases
Redox homeostasis plays a vital role in vascular function. Dysregulated redox signalling can induce endothelial dysfunction and vascular abnormalities, contributing to different types of vascular diseases, such as hypertension and atherosclerosis (AS) and the occurrence of infarction [
182,
183,
184]. Herein, we summarize the mechanisms of MnSOD-mediated protective effects through vascular remodelling in vascular diseases.
2.6. Cancer
Various aspects of the extensive role of MnSOD in cancer have been explored. MnSOD can directly mediate multiple cancer cell death signalling pathways, including apoptosis [
206], pyroptosis [
207], and autophagy [
208]. High MnSOD expression contributes to chemoresistance [
209,
210,
211] and radioresistance [
212,
213] in different cancer types. Recently, posttranslational modification of MnSOD, with particular emphasis on acetylation at lysine residue 68, was proposed and explored, which addressed its vital roles in promoting cancer progression [
214,
215,
216]. These functional roles of MnSOD in cancer have been well-reviewed elsewhere [
12,
27,
217,
218]. Herein, we summarize some emerging research hotspots in MnSOD-regulated cancer progression, with an emphasis on metabolic reprogramming and the tumour immune microenvironment.
Metabolic reprogramming has been regarded as a hallmark of cancer for centuries. The Warburg effect primarily demonstrates that malignant cells preferentially rely on glycolysis rather than oxidative phosphorylation (OXPHOS) for energy supply [
27,
219,
220]. Moreover, pathways involved in redox control are commonly reprogrammed due to tumorigenic mutations [
221]. As MnSOD is one of the major redox metabolism regulators, the effect of MnSOD on cancer metabolic reprogramming has attracted broad attention. For instance, Liu et al. reported that toll-like receptor 2 (TLR 2) induced glycolysis and metabolic shift by markedly upregulating MnSOD expression in human gastric cancer cells. Furthermore, patient-derived tissue microarrays demonstrated a significant correlation between TLR2 and MnSOD expression in gastric tumours, indicating that the TLR2-MnSOD axis could serve as a potential biomarker for therapy decisions and prognosis determination in gastric cancer [
222]. Zhou et al. observed that enhanced MnSOD expression activated AMPK signalling and shifted cell energy metabolism to glycolysis, thus facilitating tumorigenesis and progression in colorectal cancer cells [
223]. Similarly, upregulation of MnSOD in cancer cells engaged AMPK to perform and sustain the Warburg effect, therefore supporting cancer cell survival [
224]. Cells with a high level of MnSOD were more resistant to glucose-deprivation-induced cell death because MnSOD increased glucose uptake, glucose transporter member 1 (GLUT-1) availability, and OXPHOS electron transfer [
225]. These results indicate a vital role of MnSOD in cancer glycolytic metabolism reprogramming, which deserves more in-depth mechanistic studies.
As the role of the immune system in cancer development has attracted increasing attention recently, the involvement of MnSOD in the tumour immune microenvironment has also been highlighted. Accumulating evidence has suggested that tumour-infiltrating immune cells participate in cancer progression, which is highly associated with MnSOD expression in different cancer types. For example, Su et al. analysed public datasets and found that MnSOD expression is positively correlated with CXCL8 and neutrophil infiltration, indicating an involvement of the ‘MnSOD-CXCL8-neutrophil recruitment’ axis in cancer progression [
226]. MnSOD was also found to be positively correlated with CD68+ macrophage infiltration and may indicate poor outcomes in inflammation-driven lung adenocarcinoma [
227]. High MnSOD expression was observed in aggressive triple-negative breast cancer (TNBC) patients and was regarded as a poor prognostic marker. MnSOD promotes the immunosuppressive tumour microenvironment by promoting M2 macrophage invasiveness and infiltration, supporting TNBC progression [
228]. In addition, Lou et al. demonstrated that MnSOD-overexpressing oncolytic vaccinia virus (OVV-MnSOD) enhanced lymphocyte infiltration and tumour sensitivity to anti-PD-L1 treatment in lymphomatous mice, suggesting a promising therapeutic potential of MnSOD in cancer immunotherapy [
63].
In addition, we recently explored whether MnSOD overexpression could prevent cancer relapse by inhibiting reactivation of quiescent cancer cells (data not published). This novel function of MnSOD further increases the potential benefits of targeting MnSOD in cancer treatment. Although the pleiotropic role of MnSOD in cancer progression has aroused extensive interest, the underlying mechanisms remain controversial and unclear. Breakthroughs in finding key players in MnSOD-cancer regulation will be a promising research area, and will provide positive insights into the development of new drugs based on MnSOD.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232415893