Reproductive aging is on the rise globally and inseparable from the entire aging process. An extreme form of reproductive aging is premature ovarian insufficiency (POI), which to date has mostly been of idiopathic etiology, thus hampering further clinical applications and associated with enormous socioeconomic and personal costs. In the field of reproduction, timely diagnosis with a clear understanding of the various comorbidities that can arise from estrogen deficiency and the important functional role of inflammation-induced ovarian deterioration are research hotspots for appropriate counseling and preventing this prematurely ovarian aging disease. It is evident that more research is required to allow pre-emptive identification of the at-risk population and to identify mechanisms that, if addressed promptly, can prolong ovarian function and fertility.
- Reproduction
- Ovarian function
- Aging
- Premature Ovarian Insufficiency
- Infertility
1. Introduction
Premature ovarian insufficiency (POI), often referred to as premature ovarian failure (POF), is an endocrine disorder that describes a continuum of declining ovarian function, occurring in approximately every 1% of women ≥40 years of age and 0.1% of women ≥30 years of age [1][2]. In most cases, multiple factors contribute to the premature depletion of the primordial follicle pool [3]. Genetic abnormalities, autoimmunity, radiotherapy, chemotherapy, or surgery can all contribute to this disease [3][4][5][6][7]. The most common symptoms are menstrual irregularities, low-level estrogen, and high follicle-stimulating hormone (FSH); however, some POI patients have unexplained idiopathic causes [8]. POI at an early age has an undesirable impact on the reproductive system and may result in infertility. Furthermore, estrogen deficiency is linked to low bone density, cardiovascular diseases, sexual dysfunction, and high mental distress [9][10]. Though POI is a polygenic disease [11], the precise etiology and molecular mechanisms of POI remain unknown.
In recent years, the concept of aging as a disease has not prevailed in academia, but despite differences in preexisting concepts, it may not have the full symptoms of the specific disease, but still has inherent pathological features [12]. Likewise, aging has long been shown to be a major risk factor and more importantly associated with the faster progression of various health disorders [13][14]. Reproductive aging, which mostly develops over time, is a natural phenomenon [15]. Several studies have revealed the complexity and serendipitous uncertainty of the interconnectedness between reproductive aging and other organ aging [16]. Recently, numerous studies have described a strong connotation amongst inflammatory aging and POI [17][18]. In addition, ovarian biopsies of samples collected from POI patients showed lymphocytic infiltration and immune responses in the ovaries [19][20]. In the reproduction field, the significant functional role of inflammation-causing ovarian deterioration along with therapeutic approaches in order to prevent ovarian aging and increase their functionality are current research hotspots. However, further exploring the cause of POI and its association with aging needs to better understand the process of oocyte maturation and in what way the aging and inherited genetic defect are affecting it. Therefore, expanding our understanding of the disease's pathogenesis and mechanisms may be beneficial in developing an innovative strategy for treating this premature ovarian condition and preventing infertility.
2. Premature Ovarian Insufficiency or Aging
Premature ovarian insufficiency, aging, or failure (POI, POA, or POF) refers to the loss of ovarian function in a woman at a younger age than the estimates for menopausal age [1][2]. POF is one of the leading but often overlooked cause of female infertility, in which a woman’s eggs are less likely to be fertilized, and may not even be fertilized. With premature ovarian failure condition, women are still having regular menstrual cycles as well as facing difficulty in conceiving, and is seen in young women, with about 10% experiencing fertility problems. This condition of early ovarian failure is referred by Center for Human Reproduction (CHR) researchers as a clinical term of premature ovarian aging (POA) [21], which in vitro fertilization (IVF) centers also refer to as primary ovarian insufficiency (POI).
Normally, the ovaries stop functioning and decline earlier than other organs in the body, which in turn ushers in the onset of systemic aging [22]. Due to modern society’s population structure and the delay of the age of first childbearing, ovarian aging and related issues are becoming increasingly serious. Along with the substantial extension of women’s life span in modern society, the asynchronous aging of the ovary and the whole body becomes in sharp conflict with pursuing better physical and psychological well-being in women at an advanced age. In the modern era, when infertility is regarded as one of the three most prevalent diseases affecting humans, infertility rates are as high as 15–20% in developed countries. In recent years, it has been reported that the rate of female infertility in China has increased from 3% to 15–18%, and approximately 40% are related to ovarian aging. Across the entire population of women of reproductive age, 1% to 3% of women reach pathological decline in ovarian function or pathological menopause before the age of 40, also known as premature ovarian failure (POF) [23][24]. Ovarian aging has emerged as a significant threat to women’s health in modern society as a result of rising rates of infertility and longevity. Therefore, delaying ovarian aging is the key to preserving fertility in older women, thereby improving their health and quality of life.
3. Pathophysiology of Premature Ovarian Insufficiency
The ovary is the most important female reproductive organ, performing both gametogenic and secretory functions [25][26]. A healthy ovary is necessary for proper functions and for the production of sex hormones [27], and this helps females regulate their hormonal growth, reproductive cycle, and menstruation throughout their lives. Ovarian dysregulation can have an impact on a woman’s physiological or reproductive functions. The primary constituents of follicles are granulosa cells (GCs) and oocytes, which are essential for ovarian functioning [28][29]. In addition to controlling oocyte growth through the secretion of growth factors and hormones, GCs are important in maintaining follicular evolution. Hormone receptor expression in GCs, for example, follicle-stimulating hormone receptors (FSHR), is critical in ovulation as well as in folliculogenesis. Interestingly, the number of operational oocytes demonstrates a female’s reproductive capacity [30]. During folliculogenesis, the mature oocytes accompanied by GCs can interact with relevant growth hormones/factors, such as bone morphogenetic proteins (BMPs) and FSH. In most cases, even though the cause of POI is unknown, expedited GCs and oocyte apoptosis obstruct follicle maturation, anomalies in follicle activation are key mechanisms of POI in general, and the hypothalamic–pituitary–ovarian axis regulates ovarian function [31]. The primary ovarian hormones that regulate folliculogenesis are FSH and LH. Furthermore, GCs secrete anti-Müllerian hormone (AMH) during the primordial follicle phase and initial phase of antral follicles. As a result, AMH plus estrogen has a negative effect on follicular growth and influences FSH levels. Once GCs are disrupted through chemotherapy or other treatments, estrogen and AMH levels decrease, causing FSH to rise and the follicular pool to be depleted [32][33]. POI is detected by a decrease in estrogen and AMH secretion and a rise in FSH secretion. Insights into the mechanisms underlying ovarian dysfunction and follicular pool depletion can thus aid in the identification and development of effective treatments for ovarian dysfunction [34][35]. The normal folliculogenesis and pathological symptoms of POF are presented in the Figure 1.
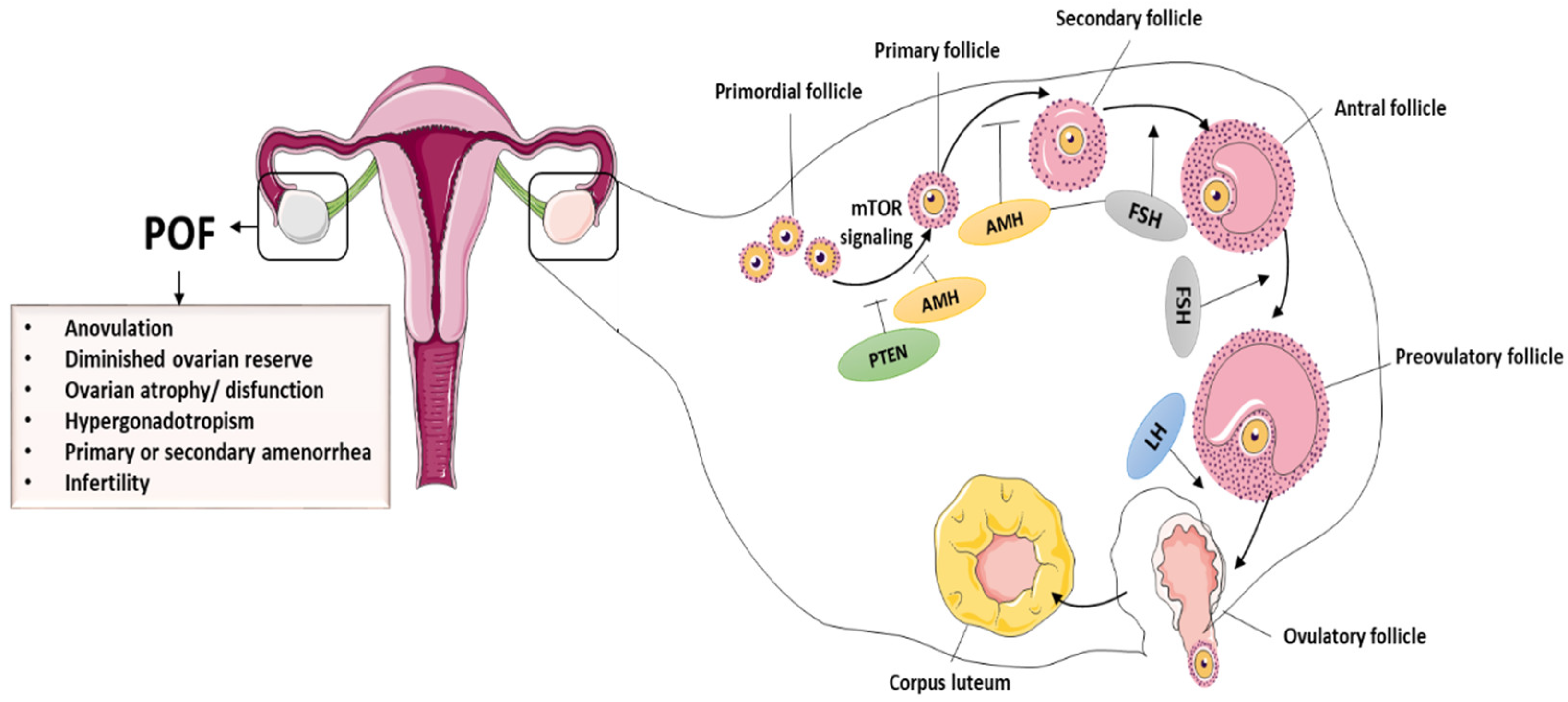
4. Causes of Premature Ovarian Insufficiency
POI is accepted in clinical practice in females under the age of 40 who show sex hormone deficiency, amenorrhea, and serum FSH level greater than 40 IU/L [36]. POI results in low levels of progesterone and estrogen. If untreated, it increases the risk of cardiovascular disease, osteoporosis, and cognitive issues. The beginning of cycle irregularity and the final cessation of menstruation is determined by the age-related decrease in follicle numbers. Natural sterility and the gradual decline in fertility are both caused by the parallel decline in oocyte quality. The decrease in negative feedback from ovarian factors at the hypothalamic–pituitary unit is the primary cause of endocrine changes. FSH levels rise first as the number of antral follicles decreases with age, then there are stages of overt cycle irregularity. Decreased levels of AMH best illustrate the gradual shrinkage of the antral follicle cohort.
Pathophysiological ovary conditions can result in follicle destruction or follicular abnormalities in cases of iatrogenic treatments, autoimmunity, genetic abnormalities, and environmental issues, and cause metabolic abnormality and infertility. Genetic factors contribute to approximately 20–25% of POI cases, where chromosomal abnormalities and genetic mutations are responsible. Multiple relevant genes have been implicated in ancestral POF, and about 14% of these cases have a positive POI genetic history [37]. Atypical chromosomal organization and mutations in particular genes linked to ovarian function or metabolic control are two examples of the genetic reasons for follicular dysfunction [38]. The DNA may also be damaged by chemotherapy and radiation therapy, which might eventually affect ovarian function by inducing apoptosis in GCs and loss of preantral follicles [39][40]. Follicular atresia is brought on by GC malfunction, and has been identified as the primary factor in POI [41]. In individuals with biochemical POI, it was shown that granulosa cell-associated transcript 1 is downregulated in GCs, demonstrating a unique lncRNA approach that promotes epigenetic control of GC activity and aids in the etiology of POI [22]. Furthermore, POI has been associated with many autoimmune diseases [8]. The autoimmune attack’s damage to ovarian function might be the underlying pathogenic factor. Even so, the exact function of the autoimmune response in ovarian dysfunction requires further investigation. However, a majority of POI cases are idiopathic [39], which encourages future research to fully comprehend this condition and investigate novel solutions.
In cases of idiopathic POI, the understanding of possible causative genetic defects and the relationship of aging with POI remains to be elucidated. Recently, numerous studies have described a strong connotation between inflammatory aging and POI [17][18]. In addition, ovarian biopsies of samples collected from POI patients showed lymphocytic infiltration and immune responses in the ovaries [19][20]. However, exploring the causes of POI and its association with aging needs to gain an understanding of the process of oocyte maturation and in what way aging is affecting it. Until now, limited research has been carried out on oocyte maturation because of research restrictions and rarer approaches to the embryos of humans with inherited POI. A variety of diseases are linked to POI, even in instances with a known origin, showing heterogeneity of this entity, and thus the identification of the exact causative defect is essential. This fact emphasizes the necessity of creating several ways to enhance the clinical care of these patients, as well as the significance of choosing the appropriate demographic of POI patients who can profit from each strategy. As a result, it is critical to investigate efficient treatment for managing POF and associated complications. Specific causes of POF require specific treatments according to the guidelines of the European Society of Human Reproduction and Embryology (ESHRE). ESHRE recommends HRT for POI treatment under conditions with hypoestrogenic symptoms [42]. Despite HRT being the standard clinical treatment for POI, it fails to restore ovarian function completely. Other treatment options, such as in vitro follicle activation, oocyte cryopreservation, and ovarian tissue transplantation, have also been investigated [43]. However, these methods have not yet been used in medical applications. The reasons are inefficient follicle activation and technical, ethical, and administrative constraints. Rapid advancements in regenerative medicine hold great potential to restore ovarian function using stem cells. Figure 2 shows the likely causes of and available treatments for POI.
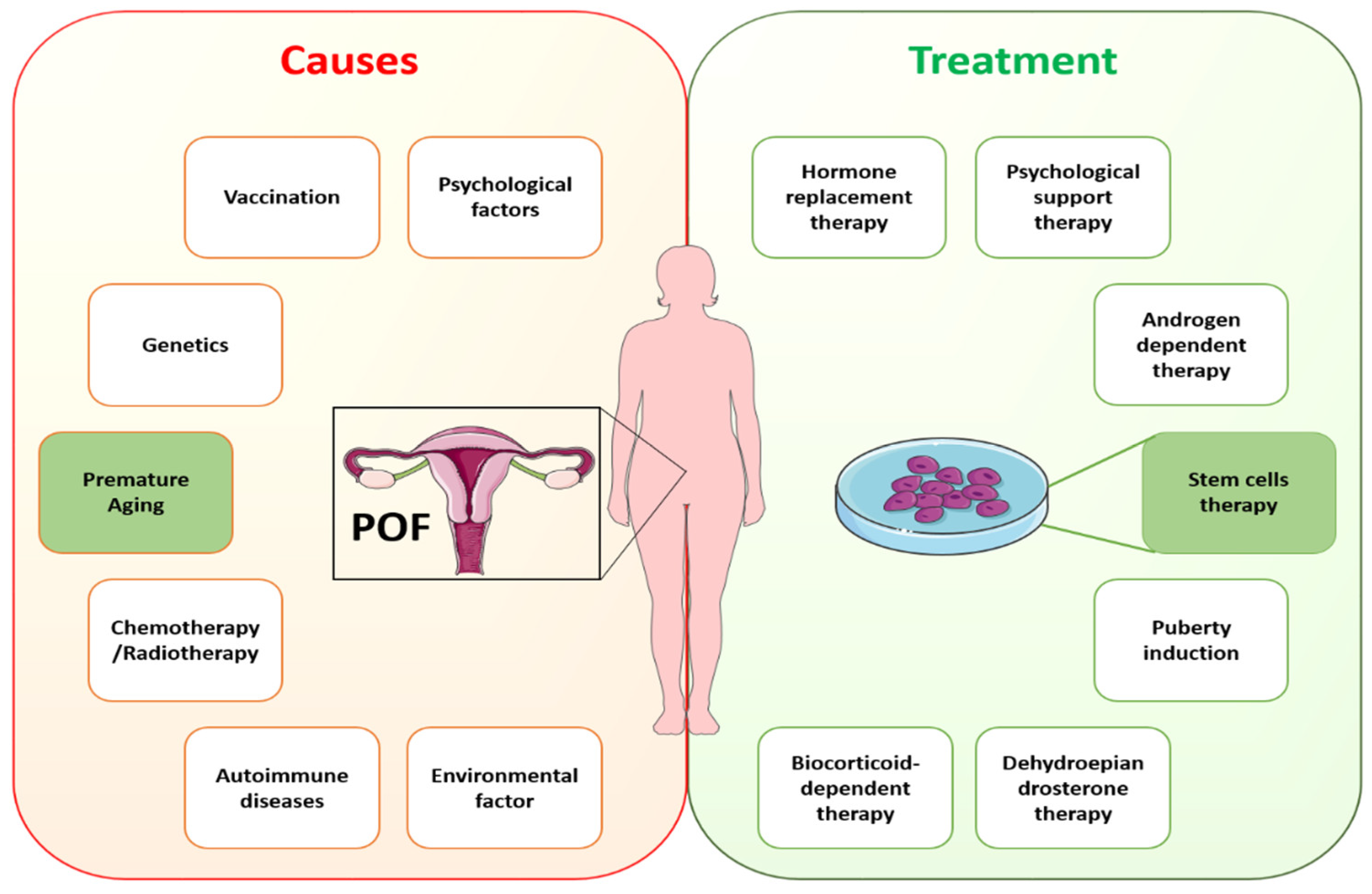
5. Aging Hallmarks of Premature Ovarian Insufficiency
There are obvious limitations to the existing information and understanding of female reproductive aging. Researchers are aware that ovarian changes play a major role in this process, which ends with menstrual cyclicity because of the ongoing loss of follicles and their eventual depletion. Alterations in ovarian feedback are considered responsible for changes in the neuroendocrine control of cyclic ovarian function. Antral follicle counts and AMH currently provide the most accurate representation of periosteal follicle loss, though these markers may not completely capture the entire decline. In addition to the decrease in quantity of oocytes, the parallel decline in oocyte competence and its effect on female fertility with age have been recognized. Aneuploid embryos are becoming more common as a result of the quality loss in oocytes, but the precise molecular biology mechanisms are still poorly understood. There is a great deal of variation among women when it comes to both the quantity and quality of ovarian decline aspects of aging. This variation suggests that many women face a clearly shorter fertility life expectancy.
The underlying mechanisms of ovarian aging remain poorly understood, especially since it is a complex biological process in which many factors interact internally and externally. The combination of internal and external elements during the body’s degeneration stage causes the long and complicated biological process of natural aging in humans. Structures deteriorate with age, the internal environment becomes imbalanced, function declines, and adaptation, resilience, and resistance are lost [44]. The study of aging is entering a new phase of inquiry due to the rapid advancement of technology, although the precise process behind aging has not yet been fully understood. Inflammation has been linked to aging in a large number of reports. Aging is followed by a persistent and developing proinflammatory condition in the body tissue and organs [45][46][47]. Figure 3 shows the important aging hallmarks of premature ovarian insufficiency.
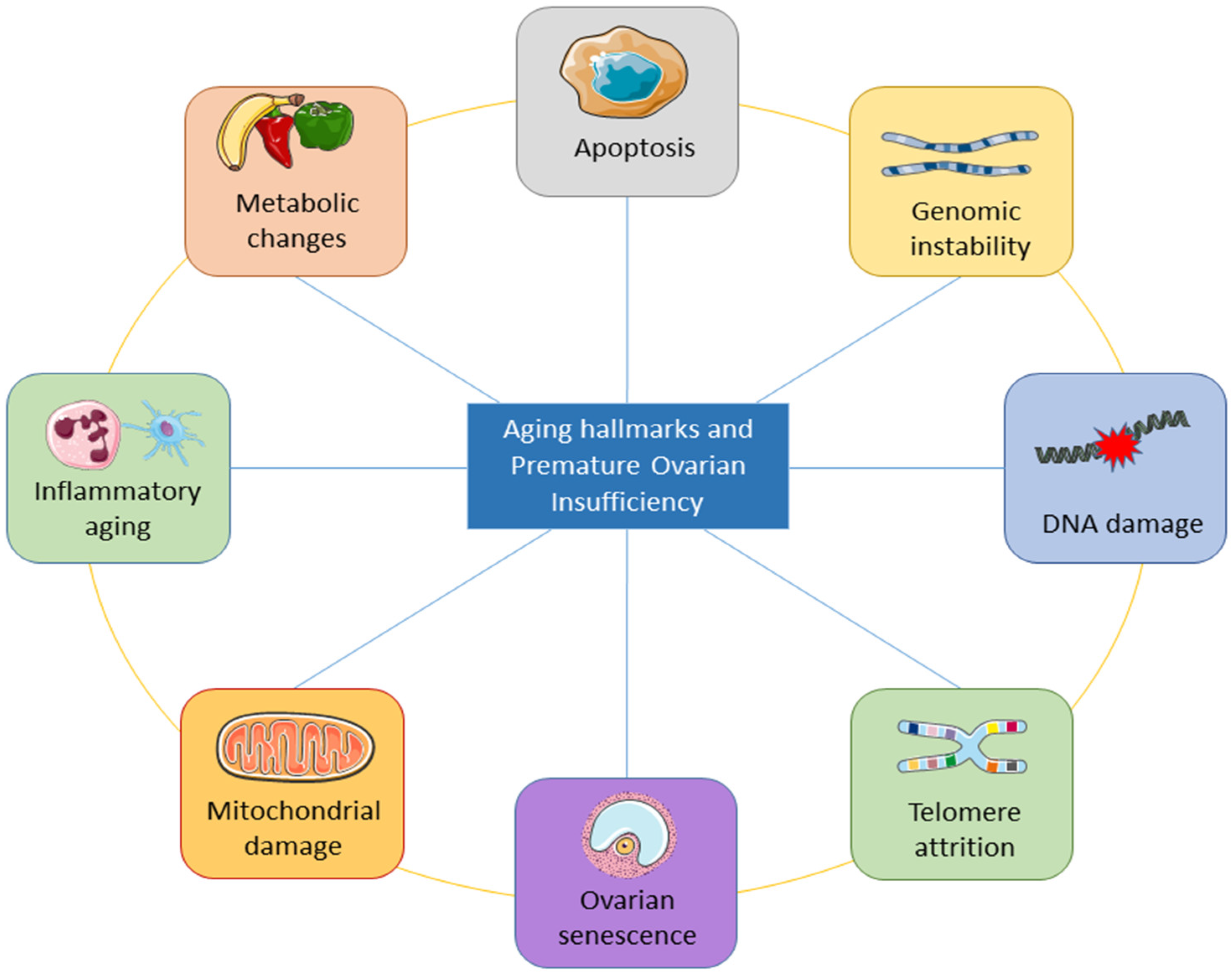
6. Premature Ovarian Aging and Infertility
Female fertility is negatively affected by premature ovarian failure due to insufficient egg numbers and poor egg quality [16]. A few low-quality eggs can markedly reduce the fertility rate of women in two ways: lowered fertility, or in cases of pregnancy, miscarriages usually occur. Unluckily, egg quality is inversely proportional to egg quantity [17][18]. As a result, females with untreated reduced ovarian reserve have the highest rates of miscarriages of any infertility diagnosis, as approximately 95% of quality embryos come from eggs, making miscarriages more likely due to poor embryo quality. However, deducing the exact mechanism of premature aging requires better understanding of the process of oocyte maturation and in what way aging and inherited genetic defects are affecting it. Limited research has been carried out on oocyte maturation because of the restrictions imposed on such research and rarer approaches to the embryos of women with inherited POI. Therefore, there is an urgent need for an effective POI model to deepen our knowledge of the pathogenesis and mechanisms of premature aging and to develop an innovative strategy for treating this ovarian aging disease and preventing infertility.
This entry is adapted from the peer-reviewed paper 10.3390/cells11233713
References
- Torrealday, S.; Kodaman, P.; Pal, L. Premature Ovarian Insufficiency—An update on recent advances in understanding and management. F1000Research 2017, 6, 2069.
- Chon, S.J.; Umair, Z.; Yoon, M.-S. Premature ovarian insufficiency: Past, present, and future. Front. Cell Dev. Biol. 2021, 10, 672890.
- Maclaren, N.; Chen, Q.-Y.; Kukreja, A.; Marker, J.; Zhang, C.H.; Sun, Z.S. Autoimmune hypogonadism as part of an autoimmune polyglandular syndrome. J. Soc. Gynecol. Investig. 2001, 8, S52–S54.
- Makin, S. Cracking the genetic code of autoimmune disease. Nature 2021, 595, 57–59.
- Woad, K.J.; Watkins, W.J.; Prendergast, D.; Shelling, A.N. The genetic basis of premature ovarian failure. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 242–244.
- Jiao, X.; Ke, H.; Qin, Y.; Chen, Z.-J. Molecular genetics of premature ovarian insufficiency. Trends Endocrinol. Metab. 2018, 29, 795–807.
- Di-Battista, A.; Moysés-Oliveira, M.; Melaragno, M.I. Genetics of premature ovarian insufficiency and the association with X-autosome translocations. Reproduction 2020, 160, R55–R64.
- Kirshenbaum, M.; Orvieto, R. Premature ovarian insufficiency (POI) and autoimmunity-an update appraisal. J. Assist. Reprod. Genet. 2019, 36, 2207–2215.
- Jiao, X.; Zhang, H.; Ke, H.; Zhang, J.; Cheng, L.; Liu, Y.; Qin, Y.; Chen, Z.-J. Premature ovarian insufficiency: Phenotypic characterization within different etiologies. J. Clin. Endocrinol. Metab. 2017, 102, 2281–2290.
- Wesevich, V.; Kellen, A.N.; Pal, L. Recent advances in understanding primary ovarian insufficiency. F1000Research 2020, 9, 1101.
- Rudnicka, E.; Kruszewska, J.; Klicka, K.; Kowalczyk, J.; Grymowicz, M.; Skórska, J.; Pięta, W.; Smolarczyk, R. Premature ovarian insufficiency–aetiopathology, epidemiology, and diagnostic evaluation. Menopause Rev./Przegląd Menopauzalny 2018, 17, 105–108.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Fedintsev, A.; Moskalev, A. Stochastic non-enzymatic modification of long-lived macromolecules-A missing hallmark of aging. Ageing Res. Rev. 2020, 62, 101097.
- Moskalev, A. Is aging a disease? Geneticist'point of view. Adv. Gerontol./Uspekhi Gerontol. 2017, 30, 843–844.
- Annelien, C.; Broekmans, F.J.; Lambalk, C.B. Role of AMH in Prediction of Menopause. Clinical Applications of Anti-Mullerian Hormone and its Measurement in Reproductive Medicine and Women’s Health. Front. Endocrinol. 2022, 12, 1664–2392.
- Yureneva, S.; Averkova, V.; Silachev, D.; Donnikov, A.; Gavisova, A.; Serov, V.; Sukhikh, G. Searching for female reproductive aging and longevity biomarkers. Aging 2021, 13, 16873.
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.; Zheng, Y. Inflamm-aging: A new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019, 2019, 8069898.
- Mason, J.B.; Habermehl, T.L.; Underwood, K.B.; Schneider, A.; Brieño-Enriquez, M.A.; Masternak, M.M.; Parkinson, K.C. The interrelationship between female reproductive aging and survival. J. Gerontol. Ser. A 2022, 77, 75–83.
- Sen, A.; Kushnir, V.A.; Barad, D.H.; Gleicher, N. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 2014, 10, 37–50.
- Reato, G.; Morlin, L.; Chen, S.; Furmaniak, J.; Smith, B.R.; Masiero, S.; Albergoni, M.; Cervato, S.; Zanchetta, R.; Betterle, C. Premature ovarian failure in patients with autoimmune Addison's disease: Clinical, genetic, and immunological evaluation. J. Clin. Endocrinol. Metab. 2011, 96, E1255–E1261.
- Sükür, Y.E.; Kıvançlı, I.B.; Ozmen, B. Ovarian aging and premature ovarian failure. J. Turk. Ger. Gynecol. Assoc. 2014, 15, 190–196.
- Li, D.; Wang, X.; Dang, Y.; Zhang, X.; Zhao, S.; Lu, G.; Chan, W.-Y.; Leung, P.C.; Qin, Y. lncRNA GCAT1 is involved in premature ovarian insufficiency by regulating p27 translation in GCs via competitive binding to PTBP1. Mol. Ther.-Nucleic Acids 2021, 23, 132–141.
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte aging underlies female reproductive aging: Biological mechanisms and therapeutic strategies. Reprod. Med. Biol. 2015, 14, 159–169.
- Ruth, K.S.; Day, F.R.; Hussain, J.; Martínez-Marchal, A.; Aiken, C.E.; Azad, A.; Thompson, D.J.; Knoblochova, L.; Abe, H.; Tarry-Adkins, J.L. Genetic insights into biological mechanisms governing human ovarian ageing. Nature 2021, 596, 393–397.
- Saitou, M.; Hayashi, K. Mammalian in vitro gametogenesis. Science 2021, 374, eaaz6830.
- Edson, M.A.; Nagaraja, A.K.; Matzuk, M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009, 30, 624–712.
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214.
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157.
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360.
- Chen, D.; Liu, W.; Lukianchikov, A.; Hancock, G.V.; Zimmerman, J.; Lowe, M.G.; Kim, R.; Galic, Z.; Irie, N.; Surani, M.A. Germline competency of human embryonic stem cells depends on eomesodermin. Biol. Reprod. 2017, 97, 850–861.
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303.
- Williams, C.J.; Erickson, G.F. Morphology and Physiology of the Ovary; MDText.com, Inc.: South Dartmouth, MA, USA, 2015.
- Dodson, W.C.; Whitesides, D.B.; Hughes, C.L., Jr.; Easley, H., III; Haney, A. Superovulation with intrauterine insemination in the treatment of infertility: A possible alternative to gamete intrafallopian transfer and in vitro fertilization. Fertil. Steril. 1987, 48, 441–445.
- Domniz, N.; Meirow, D. Premature ovarian insufficiency and autoimmune diseases. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 60, 42–55.
- Fortuño, C.; Labarta, E. Genetics of primary ovarian insufficiency: A review. J. Assist. Reprod. Genet. 2014, 31, 1573–1585.
- Vujovic, S. Aetiology of premature ovarian failure. Menopause Int. 2009, 15, 72–75.
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423.
- Chapman, C.; Cree, L.; Shelling, A.N. The genetics of premature ovarian failure: Current perspectives. Int. J. Women's Health 2015, 7, 799.
- Sheikhansari, G.; Aghebati-Maleki, L.; Nouri, M.; Jadidi-Niaragh, F.; Yousefi, M. Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomed. Pharmacother. 2018, 102, 254–262.
- Mark-Kappeler, C.J.; Hoyer, P.B.; Devine, P.J. Xenobiotic effects on ovarian preantral follicles. Biol. Reprod. 2011, 85, 871–883.
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316.
- Gelbaya, T.; Vitthala, S.; Nardo, L.; Seif, M. Optimizing hormone therapy for future reproductive performance in women with premature ovarian failure. Gynecol. Endocrinol. 2011, 27, 1–7.
- Haller-Kikkatalo, K.; Uibo, R.; Kurg, A.; Salumets, A. The prevalence and phenotypic characteristics of spontaneous premature ovarian failure: A general population registry-based study. Hum. Reprod. 2015, 30, 1229–1238.
- Vohra, B.; Sharma, S.; Kansal, V. Age-dependent variations in mitochondrial and cytosolic antioxidant enzymes and lipid peroxidation in different regions of central nervous system of guinea pigs. Indian J. Biochem. Biophys. 2001, 38, 321–326.
- Prattichizzo, F.; Micolucci, L.; Cricca, M.; De Carolis, S.; Mensà, E.; Ceriello, A.; Procopio, A.D.; Bonafè, M.; Olivieri, F. Exosome-based immunomodulation during aging: A nano-perspective on inflamm-aging. Mech. Ageing Dev. 2017, 168, 44–53.
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466.e454.
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-kB signaling is the molecular culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105.
