Wearable electronics are gaining popularity as a platform for the next generation of human-friendly electronic devices. Therefore, a new class of devices with various functionality and amenability for the human body is essential. Traditional textile materials, such as fiber, yarn, and fabric, are non-conductive. Innovative methods and novel processing technologies have been introduced to impart conductivity in textile materials to solve this issue. Coating, printing, deposition, and in situ polymerization are common techniques for this purpose. In this article, the newly developed methods with significant potential are summarized, which includes their conductivity level in different applications, such as batteries, displays, and sensors.
- wearable electronics
- conductive textiles
- vapor deposition
- conductive coating
- 3D printing
1. Deposition Method
1.1. Vapor Deposition
1.2. Layer-by-Layer Deposition (LbLD)
1.3. Electrochemical Deposition
1.4. Electroless Deposition
2. In Situ Polymerization
3. Coating
3.1. Dip Coating
3.2. Rod Coating
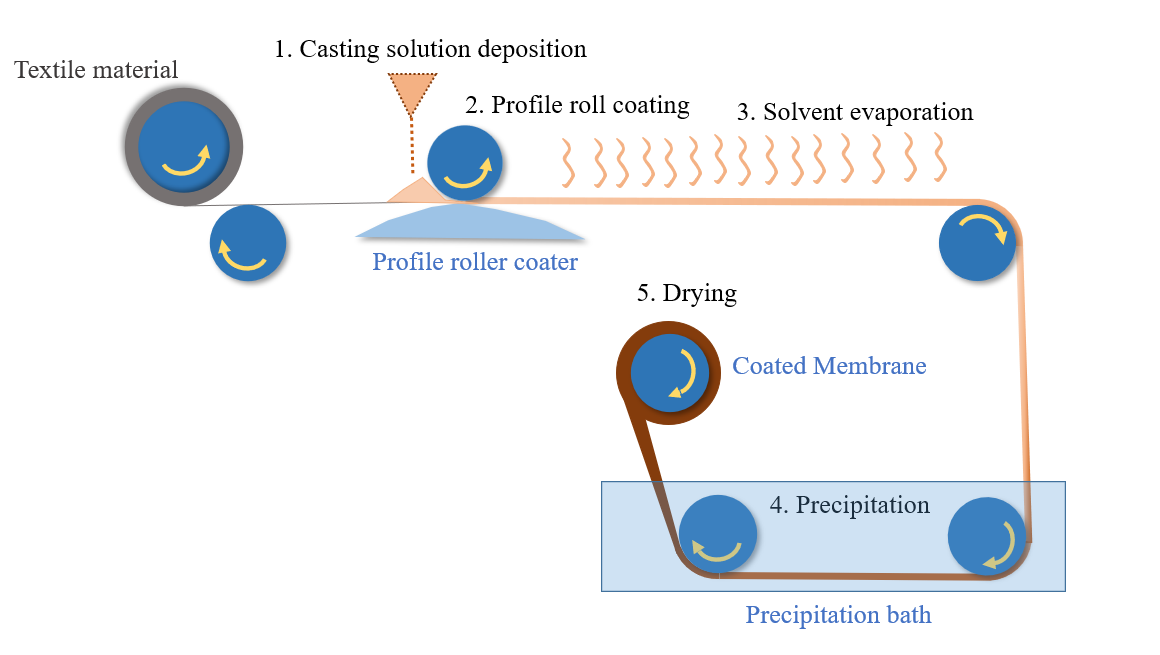
3.3. Roller Coating
4. Printing
4.1. Screen Printing
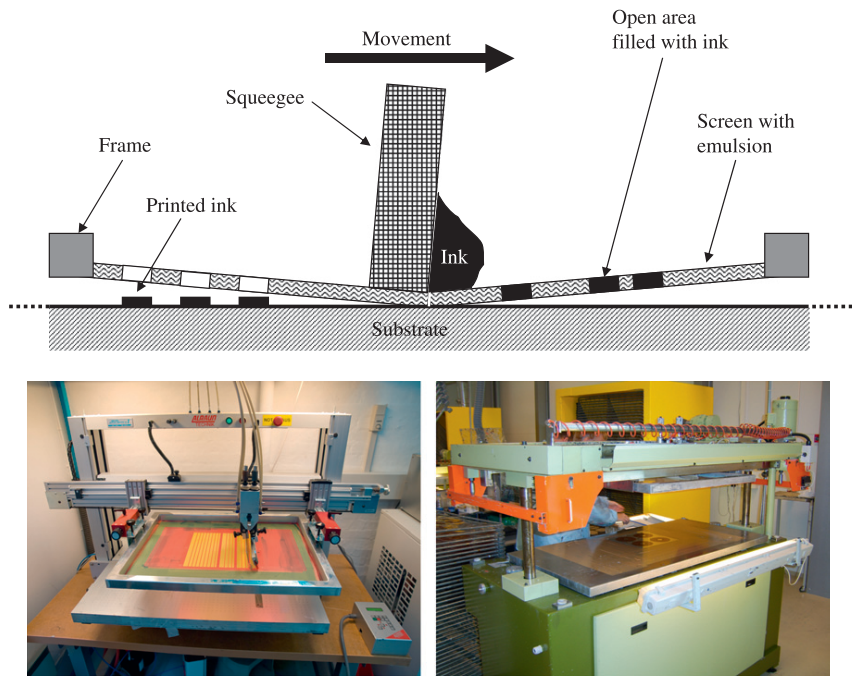
4.2. Inkjet Printing
4.3. 3D Printing
This entry is adapted from the peer-reviewed paper 10.3390/signals4010001
References
- Shahidi, S.; Moazzenchi, B.; Ghoranneviss, M. A review-application of physical vapor deposition (PVD) and related methods in the textile industry. Eur. Phys. J. Appl. Phys. 2015, 71, 31302.
- Liu, J.L.; Bashir, S. Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2015.
- Filho, J.M.C.D.S.; Ermakov, V.A.; Marques, F.C. Perovskite Thin Film Synthesised from Sputtered Lead Sulphide. Sci. Rep. 2018, 8, 1–8.
- Ostroverkhova, O. Handbook of Organic Materials for Electronic and Photonic Devices, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2019.
- Pawlak, R.; Korzeniewska, E.; Koneczny, C.; Hałgas, B. Properties Of Thin Metal Layers Deposited On Textile Composites By Using The Pvd Method For Textronic Applications. Autex Res. J. 2017, 17, 229–237.
- Montazer, M.; Harifi, T. Nanofinishing of Textile Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018.
- Miśkiewicz, P.; Frydrych, I.; Cichocka, A. Application of Physical Vapor Deposition in Textile Industry. Autex Res. J. 2022, 22, 42–54.
- Shishkovsky, I.; Lebedev, P. 3—Chemical and physical vapor deposition methods for nanocoatings. In Nanocoatings and Ultra-Thin Films; Makhlouf, A.S.H., Tiginyanu, I., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 57–77.
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010.
- Silva, N.L.; Gonçalves, L.M.; Carvalho, H. Deposition of conductive materials on textile and polymeric flexible substrates. J. Mater. Sci. Mater. Electron. 2012, 24, 635–643.
- Jilani, A.; Abdel-Wahab, M.S.; Hammad, A.H. Advance Deposition Techniques for Thin Film and Coating. In Modern Technologies for Creating the Thin-Film Systems and Coatings; Nikitenkov, N.N., Ed.; IntechOpen: London, UK, 2017; pp. 137–149.
- Aghamiri, S.; Rabiee, N.; Ahmadi, S.; Rabiee, M.; Bagherzadeh, M.; Karimi, M. Microfluidic devices: Synthetic approaches. In Biomedical Applications of Microfluidic Devices; Academic Press: London, UK, 2021; pp. 23–36.
- Krishna, J.; Perumal, A.S.; Khan, I.; Chelliah, R.; Wei, S.; Swamidoss, C.M.A.; Oh, D.-H.; Bharathiraja, B. Synthesis of nanomaterials for biofuel and bioenergy applications. In Nanomaterials; Academic Press: London, UK, 2021; pp. 97–165.
- Renken, A.; Kiwi-Minsker, L. Microstructured catalytic reactors. In Advances in Catalysis; Academic Press: Amsterdam, The Netherlands, 2010; Volume 53, pp. 47–122.
- Vančo, M.; Krmela, J.; Pešlová, F. The Use of PVD Coating on Natural Textile Fibers. Procedia Eng. 2016, 136, 341–345.
- Esen, M.; Ilhan, I.; Karaaslan, M.; Esen, R. Investigation of electromagnetic and ultraviolet properties of nano-metal-coated textile surfaces. Appl. Nanosci. 2019, 10, 551–561.
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199.
- Park, J.; Lee, J.W.; Choi, H.J.; Jang, W.G.; Kim, T.S.; Suh, D.S.; Jeong, H.Y.; Chang, S.Y.; Roh, J.C.; Yoo, C.S.; et al. Electromagnetic interference shielding effectiveness of sputtered NiFe/Cu multi-layer thin film at high frequencies. Thin Solid Films 2019, 677, 130–136.
- Jang, S.; Cho, J.; Jeong, K.; Cho, G. Exploring possibilities of ECG electrodes for bio-monitoring smartwear with Cu sputtered fabrics. In Proceedings of the International Conference on Human-Computer Interaction, Beijing, China, 22–27 July 2007; pp. 1130–1137.
- Wang, K.; Huang, Y.; Wang, M.; Yu, M.; Zhu, Y.; Wu, J. PVD amorphous carbon coated 3D NiCo2O4 on carbon cloth as flexible electrode for both sodium and lithium storage. Carbon 2017, 125, 375–383.
- Zhu, Y.; Huang, Y.; Wang, M.; Wang, K.; Yu, M.; Chen, X.; Zhang, Z. Novel carbon coated core-shell heterostructure grown on carbon cloth as flexible lithium-ion battery anodes. Ceram. Int. 2018, 44, 21690–21698.
- Choy, K. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170.
- de Lodyguine, A. Illuminesenz for Incandescent Lamps. U.S. Patent US575002A, 12 January 1897. Available online: https://patents.google.com/patent/US575002A/en (accessed on 30 August 2022).
- Malinauskas, A. Chemical deposition of conducting polymers. Polymer 2001, 42, 3957–3972.
- Tan, S.N.; Ge, H. Investigation into vapour-phase formation of polypyrrole. Polymer 1996, 37, 965–968.
- Dall’Acqua, L.; Tonin, C.; Peila, R.; Ferrero, F.; Catellani, M. Performances and properties of intrinsic conductive cellulose–polypyrrole textiles. Synth. Met. 2004, 146, 213–221.
- Xia, Y.; Lu, Y. Fabrication and properties of conductive conjugated polymers/silk fibroin composite fibers. Compos. Sci. Technol. 2008, 68, 1471–1479.
- Gregory, R.; Kimbrell, W.; Kuhn, H. Electrically Conductive Non-Metallic Textile Coatings. J. Coat. Fabr. 1991, 20, 167–175.
- Jang, J.; Chang, M.; Yoon, H. Chemical Sensors Based on Highly Conductive Poly(3,4-ethylenedioxythiophene) Nanorods. Adv. Mater. 2005, 17, 1616–1620.
- Tenhaeff, W.E.; Gleason, K.K. Initiated and Oxidative Chemical Vapor Deposition of Polymeric Thin Films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992.
- Yang, X.; Shang, S.; Li, L.; Tao, X.-M.; Yan, F. Vapor phase polymerization of 3,4-ethylenedioxythiophene on flexible substrate and its application on heat generation. Polym. Adv. Technol. 2009, 22, 1049–1055.
- Bashir, T.; Skrifvars, M.; Persson, N.-K. Synthesis of high performance, conductive PEDOT-coated polyester yarns by OCVD technique. Polym. Adv. Technol. 2011, 23, 611–617.
- Hyde, K.; Dong, H.; Hinestroza, J. Effect of surface cationization on the conformal deposition of polyelectrolytes over cotton fibers. Cellulose 2007, 14, 615–623.
- Wang, Y.; Wang, W.; Qi, Q.; Xu, N.; Yu, D. Layer-by-layer assembly of PDMS-coated nickel ferrite/multiwalled carbon nanotubes/cotton fabrics for robust and durable electromagnetic interference shielding. Cellulose 2020, 27, 2829–2845.
- Parvinzadeh Gashti, M.; Alimohammadi, F.; Song, G.; Kiumarsi, A. Characterization of nanocomposite coatings on textiles: A brief review on Microscopic technology. Curr. Microsc. Contrib. Adv. Sci. Technol. 2012, 2, 1424–1437.
- Gulrajani, M.; Gupta, D. Emerging techniques for functional finishing of textiles. Indian J. Fibre Text Res. 2011, 36, 388–397.
- Parsons, G.N.; Atanasov, S.E.; Dandley, E.C.; Devine, C.K.; Gong, B.; Jur, J.S.; Lee, K.; Oldham, C.J.; Peng, Q.; Spagnola, J.C.; et al. Mechanisms and reactions during atomic layer deposition on polymers. Coord. Chem. Rev. 2013, 257, 3323–3331.
- Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Atomic layer deposition on polymer fibers and fabrics for multifunctional and electronic textiles. J. Vac. Sci. Technol. A Vac. Surfaces Films 2016, 34, 010801.
- Saetia, K.; Schnorr, J.M.; Mannarino, M.M.; Kim, S.Y.; Rutledge, G.C.; Swager, T.M.; Hammond, P.T. Spray-Layer-by-Layer Carbon Nanotube/Electrospun Fiber Electrodes for Flexible Chemiresistive Sensor Applications. Adv. Funct. Mater. 2013, 24, 492–502.
- Xu, D.; Yang, R. Efficient preparation and characterization of paraffin-based microcapsules by emulsion polymerization. J. Appl. Polym. Sci. 2019, 136, 47552.
- Tian, M.; Du, M.; Qu, L.; Chen, S.; Zhu, S.; Han, G. Electromagnetic interference shielding cotton fabrics with high electrical conductivity and electrical heating behavior via layer-by-layer self-assembly route. RSC Adv. 2017, 7, 42641–42652.
- Shakir, I.; Ali, Z.; Bae, J.; Park, J.; Kang, D.J. Layer by layer assembly of ultrathin V2O5 anchored MWCNTs and graphene on textile fabrics for fabrication of high energy density flexible supercapacitor electrodes. Nanoscale 2014, 6, 4125–4130.
- Ahmed, H.B.; Emam, H.E. Layer by layer assembly of nanosilver for high performance cotton fabrics. Fibers Polym. 2016, 17, 418–426.
- Prakash, S.; Yeom, J. Chapter 4—Advanced Fabrication Methods and Techniques. In Nanofluidics and Microfluidics; William Andrew Publishing: Waltham, MA, USA, 2014; pp. 87–170.
- Shang, S.; Zeng, W. 4—Conductive nanofibres and nanocoatings for smart textiles. In Multidisciplinary Know-How for Smart-Textiles Developers; Kirstein, T., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 92–128.
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings—A review. J. Alloy. Compd. 2017, 691, 841–859.
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundamental aspects. Adv. Colloid Interface Sci. 2002, 97, 279–317.
- Lincot, D. Electrodeposition of semiconductors. Thin Solid Films 2005, 487, 40–48.
- Bicelli, L.; Bozzini, B.; Mele, C.; D’Urzo, L. A Review of Nanostructural Aspects of Metal Electrodeposition. Int. J. Electrochem. Sci. 2008, 3, 356–408.
- Biswal, H.J.; Vundavilli, P.R.; Gupta, A. Perspective—Electrodeposition of Graphene Reinforced Metal Matrix Composites for Enhanced Mechanical and Physical Properties: A Review. J. Electrochem. Soc. 2020, 167, 146501.
- Wang, Y.; Liu, A.; Han, Y.; Li, T. Sensors based on conductive polymers and their composites: A review. Polym. Int. 2019, 69, 7–17.
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200.
- Zhang, Q.-Z.; Zhang, D.; Miao, Z.-C.; Zhang, X.-L.; Chou, S.-L. Research Progress in MnO2-Carbon Based Supercapacitor Electrode Materials. Small 2018, 14, 1702883.
- Chen, H.; Wei, Z.; Zheng, X.; Yang, S. A scalable electrodeposition route to the low-cost, versatile and controllable fabrication of perovskite solar cells. Nano Energy 2015, 15, 216–226.
- Sreedeviamma, D.K.; Remadevi, A.; Sruthi, C.V.; Pillai, S.; Peethambharan, S.K. Nickel electrodeposited textiles as wearable radar invisible fabrics. J. Ind. Eng. Chem. 2020, 88, 196–206.
- Darvishzadeh, A.; Nasouri, K. Manufacturing, modeling, and optimization of nickel-coated carbon fabric for highly efficient EMI shielding. Surf. Coat. Technol. 2021, 409, 126957.
- Zhang, H.; Wang, S.; Tian, Y.; Liu, Y.; Wen, J.; Huang, Y.; Hang, C.; Zheng, Z.; Wang, C. Electrodeposition fabrication of core shell nanowire network for highly stable transparent conductive films. Chem. Eng. J. 2020, 390, 124495.
- Habib, M.A.; Rahman, M. Analysis of Electrolyte Flow in Localized Electrochemical Deposition. Procedia Eng. 2013, 56, 766–771.
- Lu, X.; Shang, W.; Chen, G.; Wang, H.; Tan, P.; Deng, X.; Song, H.; Xu, Z.; Huang, J.; Zhou, X. Environmentally Stable, Highly Conductive, and Mechanically Robust Metallized Textiles. ACS Appl. Electron. Mater. 2021, 3, 1477–1488.
- Qi, K.; Hou, R.; Zaman, S.; Qiu, Y.; Xia, B.Y.; Duan, H. Construction of Metal–Organic Framework/Conductive Polymer Hybrid for All-Solid-State Fabric Supercapacitor. ACS Appl. Mater. Interfaces 2018, 10, 18021–18028.
- Hu, L.; Chen, W.; Xie, X.; Liu, N.; Yang, Y.; Wu, H.; Yao, Y.; Pasta, M.; Alshareef, H.N.; Cui, Y. Symmetrical MnO2–Carbon Nanotube–Textile Nanostructures for Wearable Pseudocapacitors with High Mass Loading. ACS Nano 2011, 5, 8904–8913.
- Zhang, J.; Wang, Y.; Zang, J.; Xin, G.; Yuan, Y.; Qu, X. Electrophoretic deposition of MnO2-coated carbon nanotubes on a graphite sheet as a flexible electrode for supercapacitors. Carbon 2012, 50, 5196–5202.
- Qiu, Y.; Xu, P.; Guo, B.; Cheng, Z.; Fan, H.; Yang, M.; Yang, X.; Li, J. Electrodeposition of manganese dioxide film on activated carbon paper and its application in supercapacitors with high rate capability. RSC Adv. 2014, 4, 64187–64192.
- Kim, Y.K.; Hwang, S.-H.; Kim, S.; Park, H.; Lim, S.K. ZnO nanostructure electrodeposited on flexible conductive fabric: A flexible photo-sensor. Sens. Actuators B Chem. 2017, 240, 1106–1113.
- Rosa-Ortiz, S.M.; Takshi, A. Copper Electrodeposition by Hydrogen Evolution Assisted Electroplating (HEA) for Wearable Electronics. In Proceedings of the 2020 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, HI, USA, 10–13 February 2020; pp. 1–5.
- Chen, H.; Liao, F.; Yuan, Z.; Han, X.; Xu, C. Simple and fast fabrication of conductive silver coatings on carbon fabrics via an electroless plating technique. Mater. Lett. 2017, 196, 205–208.
- Cao, S.; Pedraza, A.; Allard, L. Laser-induced microstructural changes and decomposition of aluminum nitride. J. Mater. Res. 1995, 10, 54–62.
- Staňo, M.; Fruchart, O. Magnetic nanowires and nanotubes. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2018; Volume 27, pp. 155–267.
- Lahiri, A.; Pulletikurthi, G.; Endres, F. A Review on the Electroless Deposition of Functional Materials in Ionic Liquids for Batteries and Catalysis. Front. Chem. 2019, 7, 85.
- Root, W.; Aguiló-Aguayo, N.; Pham, T.; Bechtold, T. Conductive layers through electroless deposition of copper on woven cellulose lyocell fabrics. Surf. Coat. Technol. 2018, 348, 13–21.
- Gan, X.; Wu, Y.; Liu, L.; Shen, B.; Hu, W. Electroless plating of Cu–Ni–P alloy on PET fabrics and effect of plating parameters on the properties of conductive fabrics. J. Alloy. Compd. 2008, 455, 308–313.
- Lu, Y.; Jiang, S.; Huang, Y. Ultrasonic-assisted electroless deposition of Ag on PET fabric with low silver content for EMI shielding. Surf. Coat. Technol. 2010, 204, 2829–2833.
- Montazer, M.; Allahyarzadeh, V. Electroless Plating of Silver Nanoparticles/Nanolayer on Polyester Fabric Using AgNO3/NaOH and Ammonia. Ind. Eng. Chem. Res. 2013, 52, 8436–8444.
- Liu, H.; Zhu, L.-L.; He, Y.; Cheng, B.-W. A novel method for fabricating elastic conductive polyurethane filaments by in-situ reduction of polydopamine and electroless silver plating. Mater. Des. 2017, 113, 254–263.
- Lin, X.; Wu, M.; Zhang, L.; Wang, D. Superior Stretchable Conductors by Electroless Plating of Copper on Knitted Fabrics. ACS Appl. Electron. Mater. 2019, 1, 397–406.
- Guo, R.H.; Jiang, S.Q.; Yuen, C.W.M.; Ng, M.C.F. An alternative process for electroless copper plating on polyester fabric. J. Mater. Sci. Mater. Electron. 2008, 20, 33–38.
- Kim, E.-Y.; Kong, J.-S.; An, S.-K.; Kim, H.-D. Surface modification of polymers and improvement of the adhesion between evaporated copper metal film and a polymer. I. Chemical modification of PET. J. Adhes. Sci. Technol. 2000, 14, 1119–1130.
- Lu, Y. Electroless copper plating on 3-mercaptopropyltriethoxysilane modified PET fabric challenged by ultrasonic washing. Appl. Surf. Sci. 2009, 255, 8430–8434.
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva-Acuña, C. Multi-phase microstructures drive exciton dissociation in neat semicrystalline polymeric semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722.
- Ali, A.; Baheti, V.; Vik, M.; Militky, J. Copper electroless plating of cotton fabrics after surface activation with deposition of silver and copper nanoparticles. J. Phys. Chem. Solids 2019, 137, 109181.
- Li, X.; Li, Y.; Guan, T.; Xu, F.; Sun, J. Durable, Highly Electrically Conductive Cotton Fabrics with Healable Superamphiphobicity. ACS Appl. Mater. Interfaces 2018, 10, 12042–12050.
- Hassan, Z.; Atalay, O.; Kalaoglu, F. Conductive cotton fabric using laser pre-treatment and electroless plating. J. Text. Inst. 2021, 113, 737–747.
- Grothe, J.; Kaskel, S.; Leuteritz, A. Nanocomposites and hybrid materials. In Polymer Science: A Comprehensive Reference; 10 Volume Set; Elsevier: Amsterdam, The Netherlands, 2012; pp. 177–209.
- Maity, S.; Chatterjee, A. Conductive polymer-based electro-conductive textile composites for electromagnetic interference shielding: A review. J. Ind. Text. 2016, 47, 2228–2252.
- Liu, T.; Zhang, J.; Han, W.; Zhang, J.; Ding, G.; Dong, S.; Cui, G. Review—In Situ Polymerization for Integration and Interfacial Protection Towards Solid State Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070527.
- Ansari, M.O.; Khan, M.M.; Ansari, S.A.; Raju, K.; Lee, J.; Cho, M.H. Enhanced Thermal Stability under DC Electrical Conductivity Retention and Visible Light Activity of Ag/TiO2@Polyaniline Nanocomposite Film. ACS Appl. Mater. Interfaces 2014, 6, 8124–8133.
- Park, J.H.; Ko, J.M.; Park, O.; Kim, D.-W. Capacitance properties of graphite/polypyrrole composite electrode prepared by chemical polymerization of pyrrole on graphite fiber. J. Power Sources 2002, 105, 20–25.
- Hong, K.H.; Oh, K.W.; Kang, T.J. Preparation and properties of electrically conducting textiles byin situ polymerization of poly(3,4-ethylenedioxythiophene). J. Appl. Polym. Sci. 2005, 97, 1326–1332.
- Grishina, T.R. The effect of the table salt substitute sanasol on arterial pressure, water-salt metabolism and kidney functions in experimental pathology of circulatory homeostasis. Eksp. Klin. Farmakol. 1992, 55, 21–24.
- Dey, M.; Doumenc, F.; Guerrier, B. Numerical simulation of dip-coating in the evaporative regime. Eur. Phys. J. E 2016, 39, 19.
- Gaulding, E.A.; Diroll, B.T.; Goodwin, E.D.; Vrtis, Z.J.; Kagan, C.R.; Murray, C.B. Deposition of Wafer-Scale Single-Component and Binary Nanocrystal Superlattice Thin Films Via Dip-Coating. Adv. Mater. 2015, 27, 2846–2851.
- Ceratti, D.R.; Louis, B.; Paquez, X.; Faustini, M.; Grosso, D. A New Dip Coating Method to Obtain Large-Surface Coatings with a Minimum of Solution. Adv. Mater. 2015, 27, 4958–4962.
- Roland, S.; Pellerin, C.; Bazuin, C.G.; Prud’Homme, R.E. Evolution of Small Molecule Content and Morphology with Dip-Coating Rate in Supramolecular PS–P4VP Thin Films. Macromolecules 2012, 45, 7964–7972.
- Jittavanich, K.; Clemons, C.; Kreider, K.; Aljarrah, M.; Evans, E.; Young, G. Modeling, simulation and fabrication of coated structures using the dip coating technique. Chem. Eng. Sci. 2010, 65, 6169–6180.
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2016, 81, 378–404.
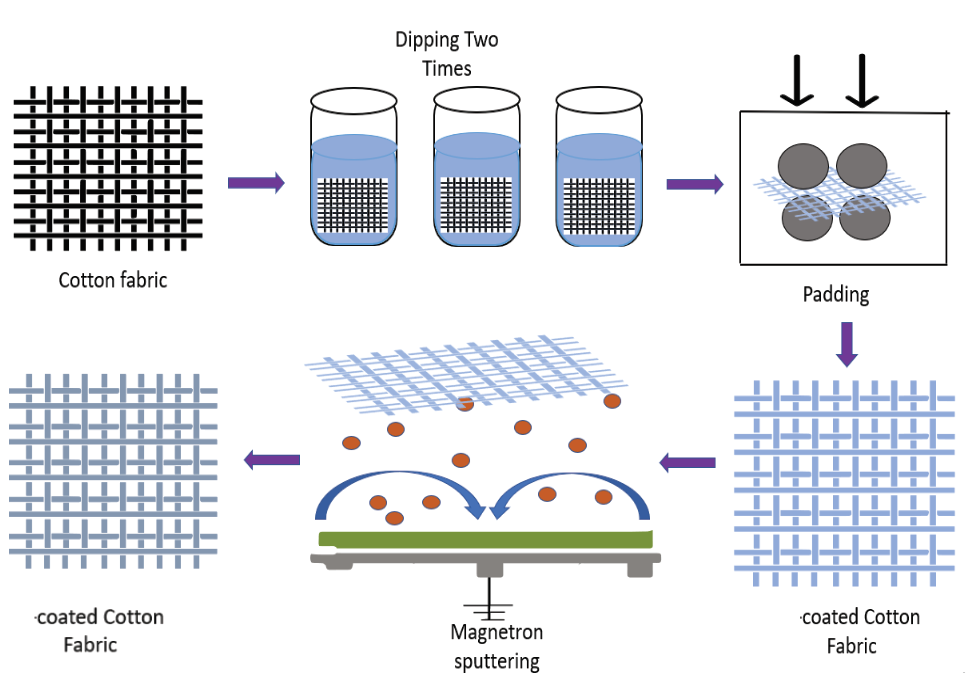
- He, S.; Xin, B.; Chen, Z.; Liu, Y. Flexible and highly conductive Ag/G-coated cotton fabric based on graphene dipping and silver magnetron sputtering. Cellulose 2018, 25, 3691–3701.
- Alongi, J.; Ciobanu, M.; Tata, J.; Carosio, F.; Malucelli, G. Thermal stability and flame retardancy of polyester, cotton, and relative blend textile fabrics subjected to sol-gel treatments. J. Appl. Polym. Sci. 2010, 119, 1961–1969.
- Gorenšek, M.; Recelj, P. Nanosilver Functionalized Cotton Fabric. Text. Res. J. 2007, 77, 138–141.
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol–gel process. J. Appl. Polym. Sci. 2007, 104, 111–117.
- Mahltig, B.; Textor, T. Combination of silica sol and dyes on textiles. J. Sol-Gel Sci. Technol. 2006, 39, 111–118.
- Schramm, C.; Binder, W.; Tessadri, R. Durable Press Finishing of Cotton Fabric with 1,2,3,4-Butanetetracarboxylic Acid and TEOS/GPTMS. J. Sol-Gel Sci. Technol. 2004, 29, 155–165.
- Kampeerapappun, P.; Visatchok, K.; Wangarsa, D. Preparation and properties of superhydrophobic cotton fabrics. J. Met. Mater. Miner. 2010, 20, 79–83.
- Nasirizadeh, N.; Dehghani, M.; Yazdanshenas, M.E. Preparation of hydrophobic and conductive cotton fabrics using multi-wall carbon nanotubes by the sol–gel method. J. Sol-Gel Sci. Technol. 2014, 73, 14–21.
- Kwon, S.; Kim, W.; Kim, H.; Choi, S.; Park, B.-C.; Kang, S.-H.; Choi, K.C. High Luminance Fiber-Based Polymer Light-Emitting Devices by a Dip-Coating Method. Adv. Electron. Mater. 2015, 1, 1500103.
- Pu, D.; Zhou, W.; Li, Y.; Chen, J.; Chen, J.; Zhang, H.; Mi, B.; Wang, L.; Ma, Y. Order-enhanced silver nanowire networks fabricated by two-step dip-coating as polymer solar cell electrodes. RSC Adv. 2015, 5, 100725–100729.
- Liu, L.; Chen, W.; Zhang, H.; Wang, Q.; Guan, F.; Yu, Z. Flexible and Multifunctional Silk Textiles with Biomimetic Leaf-Like MXene/Silver Nanowire Nanostructures for Electromagnetic Interference Shielding, Humidity Monitoring, and Self-Derived Hydrophobicity. Adv. Funct. Mater. 2019, 29, 1905197.
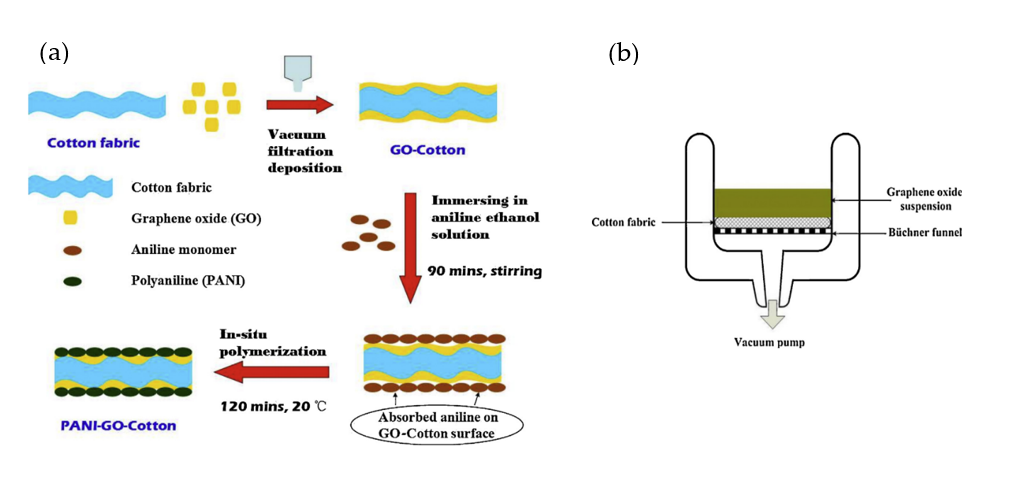
- Tang, X.; Tian, M.; Qu, L.; Zhu, S.; Guo, X.; Han, G.; Sun, K.; Hu, X.; Wang, Y.; Xu, X. Functionalization of cotton fabric with graphene oxide nanosheet and polyaniline for conductive and UV blocking properties. Synth. Met. 2015, 202, 82–88.
- Zhang, S.; Li, Y.; Pan, N. Graphene based supercapacitor fabricated by vacuum filtration deposition. J. Power Sources 2012, 206, 476–482.
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278.
- Gong, F.; Meng, C.; He, J.; Dong, X. Fabrication of highly conductive and multifunctional polyester fabrics by spray-coating with PEDOT:PSS solutions. Prog. Org. Coat. 2018, 121, 89–96.
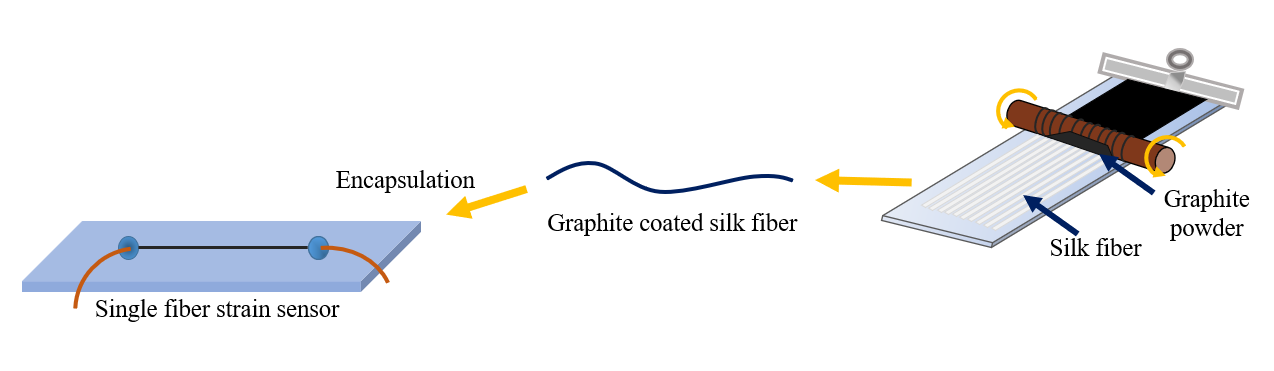
- Zhang, M.; Wang, C.; Wang, Q.; Jian, M.; Zhang, Y. Sheath–Core Graphite/Silk Fiber Made by Dry-Meyer-Rod-Coating for Wearable Strain Sensors. ACS Appl. Mater. Interfaces 2016, 8, 20894–20899.
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Carbon nanotube polymer coatings for textile yarns with good strain sensing capability. Sens. Actuators A Phys. 2012, 179, 83–91.
- Yang, K.; Torah, R.; Wei, Y.; Beeby, S.; Tudor, J. Waterproof and durable screen printed silver conductive tracks on textiles. Text. Res. J. 2013, 83, 2023–2031.
- Paul, G.; Torah, R.; Yang, K.; Beeby, S.; Tudor, J. An investigation into the durability of screen-printed conductive tracks on textiles. Meas. Sci. Technol. 2014, 25, 025006.
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412.
- Virkki, J.; Björninen, T.; Merilampi, S.; Sydänheimo, L.; Ukkonen, L. The effects of recurrent stretching on the performance of electro-textile and screen-printed ultra-high-frequency radio-frequency identification tags. Text. Res. J. 2014, 85, 294–301.
- Yang, Y.-L.; Chuang, M.-C.; Lou, S.-L.; Wang, J. Thick-film textile-based amperometric sensors and biosensors. Analyst 2010, 135, 1230–1234.
- Sadi, S.; Yang, M.; Luo, L.; Cheng, D.; Cai, G.; Wang, X. Direct screen printing of single-faced conductive cotton fabrics for strain sensing, electrical heating and color changing. Cellulose 2019, 26, 6179–6188.
- Torah, R.; Wei, Y.; Li, Y.; Yang, K.; Beeby, S.; Tudor, J. Printed textile-based electronic devices. In Handbook of Smart Textiles; Springer: Singapore, 2015; pp. 653–687.
- Fukuda, K.; Someya, T. Recent Progress in the Development of Printed Thin-Film Transistors and Circuits with High-Resolution Printing Technology. Adv. Mater. 2016, 29, 1602736.
- Matavž, A.; Bobnar, V.; Malič, B. Tailoring Ink–Substrate Interactions via Thin Polymeric Layers for High-Resolution Printing. Langmuir 2017, 33, 11893–11900.
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing-Process and Its Applications. Adv. Mater. 2010, 22, 673–685.
- Lim, J.A.; Lee, W.H.; Lee, H.S.; Lee, J.H.; Park, Y.D.; Cho, K. Self-Organization of Ink-jet-Printed Triisopropylsilylethynyl Pentacene via Evaporation-Induced Flows in a Drying Droplet. Adv. Funct. Mater. 2008, 18, 229–234.
- Kim, I.; Shahariar, H.; Ingram, W.F.; Zhou, Y.; Jur, J.S. Inkjet Process for Conductive Patterning on Textiles: Maintaining Inherent Stretchability and Breathability in Knit Structures. Adv. Funct. Mater. 2018, 29, 1807573.
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll fabrication of large area functional organic materials. J. Polym. Sci. Part B Polym. Phys. 2012, 51, 16–34.
- Karim, N.; Afroj, S.; Malandraki, A.; Butterworth, S.; Beach, C.; Rigout, M.; Novoselov, K.S.; Casson, A.J.; Yeates, S.G. All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. C 2017, 5, 11640–11648.
- Beecroft, M. 3D printing of weft knitted textile based structures by selective laser sintering of nylon powder. IOP Conf. Ser. Mater. Sci. Eng. 2016, 137, 012017.
- Hamzah, H.H.; Shafiee, S.A.; Abdalla, A.; Patel, B.A. 3D printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem. Commun. 2018, 96, 27–31.
- Dip, T.M.; Emu, A.S.; Nafiz, M.N.H.; Kundu, P.; Rakhi, H.R.; Sayam, A.; Akhtarujjman, M.; Shoaib, M.; Ahmed, M.S.; Ushno, S.T.; et al. 3D printing technology for textiles and fashion. Text. Prog. 2021, 52, 167–260.
- Chizari, K.; Daoud, M.A.; Ravindran, A.R.; Therriault, D. 3D Printing of Highly Conductive Nanocomposites for the Functional Optimization of Liquid Sensors. Small 2016, 12, 6076–6082.
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2015, 26, 698–703.
- Gnanasekaran, K.; Heijmans, T.; van Bennekom, S.; Woldhuis, H.; Wijnia, S.; de With, G.; Friedrich, H. 3D printing of CNT- and graphene-based conductive polymer nanocomposites by fused deposition modeling. Appl. Mater. Today 2017, 9, 21–28.
- Gao, T.; Yang, Z.; Chen, C.; Li, Y.; Fu, K.; Dai, J.; Hitz, E.M.; Xie, H.; Liu, B.; Song, J.; et al. Three-Dimensional Printed Thermal Regulation Textiles. ACS Nano 2017, 11, 11513–11520.
- He, H.; Akbari, M.; Sydänheimo, L.; Ukkonen, L.; Virkki, J. 3D-Printed Graphene Antennas and Interconnections for Textile RFID Tags: Fabrication and Reliability towards Humidity. Int. J. Antennas Propag. 2017, 2017, 1–5.
