Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Chemical
Nanofiltration (NF) is one of the promising technologies for water reclamation application, particularly in desalination, water, and wastewater treatment fields. A membrane with antifouling capability can be developed by tuning the membrane’s physicochemical properties to alter the membrane-foulant interaction.
- nanofiltration
- antifouling
- modification
- membrane
1. Introduction
It is undeniable that the world is facing increasingly severe problems with water and energy scarcity. More than 50% of the world’s population has severe water shortages, which are forecast to worsen in the coming years due to population growth [1]. This situation calls for urgent action to increase the clean water supply globally. Thus, the United Nations (UN) has established a target for water as part of the Sustainable Development Goals (SDG) agenda, namely Goal No. 6—“Ensure availability and sustainable management of water and sanitation for everyone” [2]. To accomplish this goal, desalination technology and water recycling and reuse can be implemented.
Membrane technologies have become a possible option for increasing freshwater water supplies due to their low energy requirement, small footprint, clean process, and high productivity [3]. Nanofiltration (NF) membrane is one of the promising membrane processes for desalination and wastewater treatment. NF membranes typically have a molecular weight cut-off (MWCO) of 100–2000 Da with a nominal pore size of 1 nm, allowing them to separate monovalent salts and water from multivalent salts and low-molecular weight organics [4]. Moreover, the NF process possesses a good balance between energy usage and treatment capacity [5]. Despite the unique feature of NF and its advantages, fouling susceptibility is one of the challenges to the widespread implementation of NF membranes. In actual applications, the fouling problem is typically the most crucial aspect that may define the cut-off point of the membrane’s performance [5,6]. Membrane fouling can lead to a loss of water production and membrane integrity, lower water quality, and a shorter membrane life span [7]. Cleaning up the foulant to maintain the membrane’s performance usually involves more chemicals or energy, thereby increasing operational costs. As a result, the plant operation must bear a significant financial burden [7,8]. Therefore, it is essential to address the fouling issue to ensure the effectiveness of a membrane process in water reclamation applications, particularly in desalination, water, and wastewater treatment fields.
To alleviate the fouling problem, the research and development of antifouling membranes has become an important area. Compared to other fouling mitigation strategies, such as optimizing the operating conditions, the membrane modification approach to improve the antifouling feature is preferred because it will require only minor adjustments to existing setups [9]. Several reviews related to various NF aspects have been published thus far, including the NF application in water and wastewater treatments [10,11,12], overall NF process development and trends [13,14], ceramic NF [15,16], NF for resource recovery [17], NF for water recycling and reuse [3], and NF for dye/salt separation [18]. Concerns about the antifouling membrane topic have been reflected in some reviews, most of which focus on reverse osmosis (RO) membranes [19,20,21,22]. There are still no comprehensive reviews in the past five years that concentrate on the development of antifouling NF membranes.
2. Membrane Fouling
Generally, a fouled membrane refers to a phenomenon where an undesired accumulation of solutes occurs either outwardly on the membrane surface, inside the pores (porous membrane case), or both [23]. Unlike low pressure-based membranes such as ultrafiltration (UF) and microfiltration (MF), which experience internal fouling, the NF membrane is primarily controlled by the surface fouling and scaling associated with divalent ions [22,24]. Fouling formation causes an increase in mass transfer resistance for water permeation, reducing membrane productivity.
Based on the nature of the foulants, it is possible to classify fouling as colloidal, inorganic, organic, and biofouling (Figure 1). Colloidal fouling occurs when the colloidal particles (size range of 1 nm–1 μm) adhere or deposit on the membrane surface [20]. Natural organic matter and organic and inorganic colloids, such as iron and silica, are examples of colloidal particulates. Since colloidal foulants can include both inorganic and organic elements, some sources classify colloidal fouling as part of either inorganic or organic fouling. Inorganic fouling (scaling) occurs when the level of inorganic ions in water exceeds the saturation point. This phenomenon leads inorganic ions to enter the nucleation step, forming the crystal that can deposit on the membrane surface or pores [25]. The most prevalent scalants on the membrane surface are inorganic salts with very low solubilities, such as barium sulfate (BaSO4), calcium carbonate (CaCO3), calcium sulfate (CaSO4), and silica (SiO2). Organic fouling describes the accumulation of organic matter on the membrane structure. Examples of organic matter include proteins, organic acids, nucleic acids, humic substances, and lipids [25]. Low- to medium-molecular weight organic foulants (300–1000 Da) have been observed to play a substantial role in the early phases of membrane fouling. On the other hand, high-molecular weight organic matter (> 50,000 Da) ruled the later stages of fouling layer development [26]. Biofouling, also known as proliferative fouling, involves the adhesion and proliferation of biologically active organisms on the membrane surface [27]. Biofoulants such as fungi, viruses, and bacteria create the biofilm layer. Moreover, the presence of salt in water or wastewater can stimulate the endogenous respiration and cell aggregation of bacteria, which in turn leads to an increase in the amount of extracellular polymeric substances (EPS) produced by cell secretion [28,29]. The high amount of EPS may cause the viscosity of the wastewater to rise even more and may also lead to the formation of an intractable cake layer on the surface of the membrane [30,31].
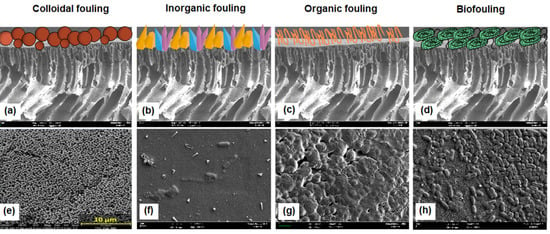
Figure 1. The schematic (a–d) and real membrane surface images (e–h) of different types of fouling (image (e) adapted from ref [32]).
A recent study by Lin et al. [33] demonstrated the intricacies of the fouling phenomena occurring during the actual two-stage module NF process. It was found that at different locations of the modules, different types of fouling predominated. Biofouling and organic-biological fouling were found to dominate at the head of the first module, while organic fouling was predominant at the head of the second module. It was concluded that the type of fouling shifted at different locations in the module due to the continuous water quality change throughout the process. Thus, NF membranes must be able to cope with different fouling types for real water and wastewater treatment applications.
Diverse strategies may be employed to reduce membrane fouling. Pre-treatment is a common method to minimize the foulants level in feed water before reaching the membrane unit. Some pre-treatment processes involve the feed pH adjustment and employ several techniques; either membrane (e.g., UF and MF) or non-membrane processes such as coagulation to remove pollutants that can exacerbate fouling [34,35,36]. In certain cases, anti-scalants can also be added to the pre-treated water before entering the membrane process but their amount needs to be regulated appropriately to prevent increased fouling accumulation caused by overdosing [37]. Besides feed water quality, hydrodynamic conditions associated with different crossflow velocities, specific transmembrane pressures, and other operating conditions such as permeate recovery rate could also influence the fouling phenomenon [7]. A membrane’s surface or pore interior can become fouled even if operating conditions and antiscalant dosage are optimised. Thus, membrane cleaning procedures such as chemical cleaning and physical cleaning (e.g., backwashing, reverse flush, and air spurge) have been implemented in the membrane process [7,23]. Even so, it is possible to lessen the frequency of these fouling mitigation measures by altering the membrane to make it less vulnerable to fouling.
A membrane with antifouling capability can be developed by tuning the membrane’s physicochemical properties to alter the membrane-foulant interaction. For instance, it is known that most foulants, including proteins, are hydrophobic by nature [38]. Thus, a hydrophilic membrane will have a lower fouling tendency since more water molecules could be adsorbed on the membrane surface, creating a hydration layer that could minimize the membrane–hydrophobic foulant interaction (Figure 2a) [20]. Typically, a hydrophilic surface is characterized by a low water contact angle (0° < Ɵ < 90°) and a higher value of Gibbs free energy [39,40]. Nevertheless, in practical applications, water contact angle measurement is considerably more frequently used for evaluating membrane surface hydrophilicity since it is simpler and easier to use. In terms of surface morphology, a smoother surface is less likely to become clogged with the foulant, whereas a rougher surface has a greater fouling propensity [41,42]. The fouling phenomenon can occur on thin-film composite (TFC) polyamide NF membranes despite their relatively hydrophilic surface. This is because the interfacial polymerization process used to fabricate the polyamide-based TFC often results in a rough membrane surface [23]. Therefore, membrane modification to reduce surface roughness can enhance the antifouling property. Meanwhile, tuning the surface charge is another approach to fabricating an antifouling membrane [22]. The fouling can be minimized by improving the electrostatic repulsion between the charged membrane surface and charged foulants. Hence, adjusting the membrane surface charge should consider the charge characteristics of targeted foulants in the water or wastewater to be treated. For instance, the negatively charged membrane is anticipated to have superior anti-biofouling efficacy since most bacteria are negatively charged at neutral pH [43].
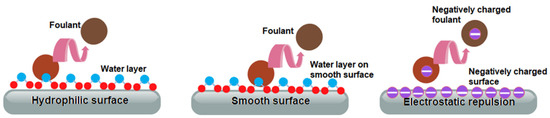
Figure 2. Illustration of membrane surface properties and antifouling mechanism.
3. Strategies for the Development of Antifouling NF Membrane
Generally, two common strategies have been identified for fabricating the antifouling NF membrane, namely (i) blending the additive or surface modifying agents with membrane casting solution (for asymmetric membranes) or blending the additive with monomer during interfacial polymerization step (for thin-film nanocomposite (TFN)) and (ii) surface modification of pre-formed membrane. Coating, grafting, covalent coupling, plasma treatment, and surface patterning are examples of surface modification techniques that have been employed in improving antifouling properties [22]. The coating technique is a straightforward process in which a thin layer of material (e.g., nanoparticles, macromolecules, and polymers) is introduced onto the membrane surface without any covalent attachment. Solution coating or layer-by-layer (LBL) techniques can create a coating layer during surface modification. A curing procedure is sometimes applied to the coating layer to ensure stability. The grafting technique is conducted by adhering the surface-modifying polymers or macromolecules to the membrane surface via various grafting mechanisms such as redox, free radical, plasma enzymatic, cationic, anionic, and atom transfer radical polymerization (ATRP). Unlike grafting, the covalent coupling method uses low-molecular weight materials to adhere to the membrane surface via interaction with reactive functional groups. Meanwhile, surface hydrophilicity or surface crosslinking can be achieved by plasma treatment or irradiation procedures [22].
This entry is adapted from the peer-reviewed paper 10.3390/membranes12121276
This entry is offline, you can click here to edit this entry!
