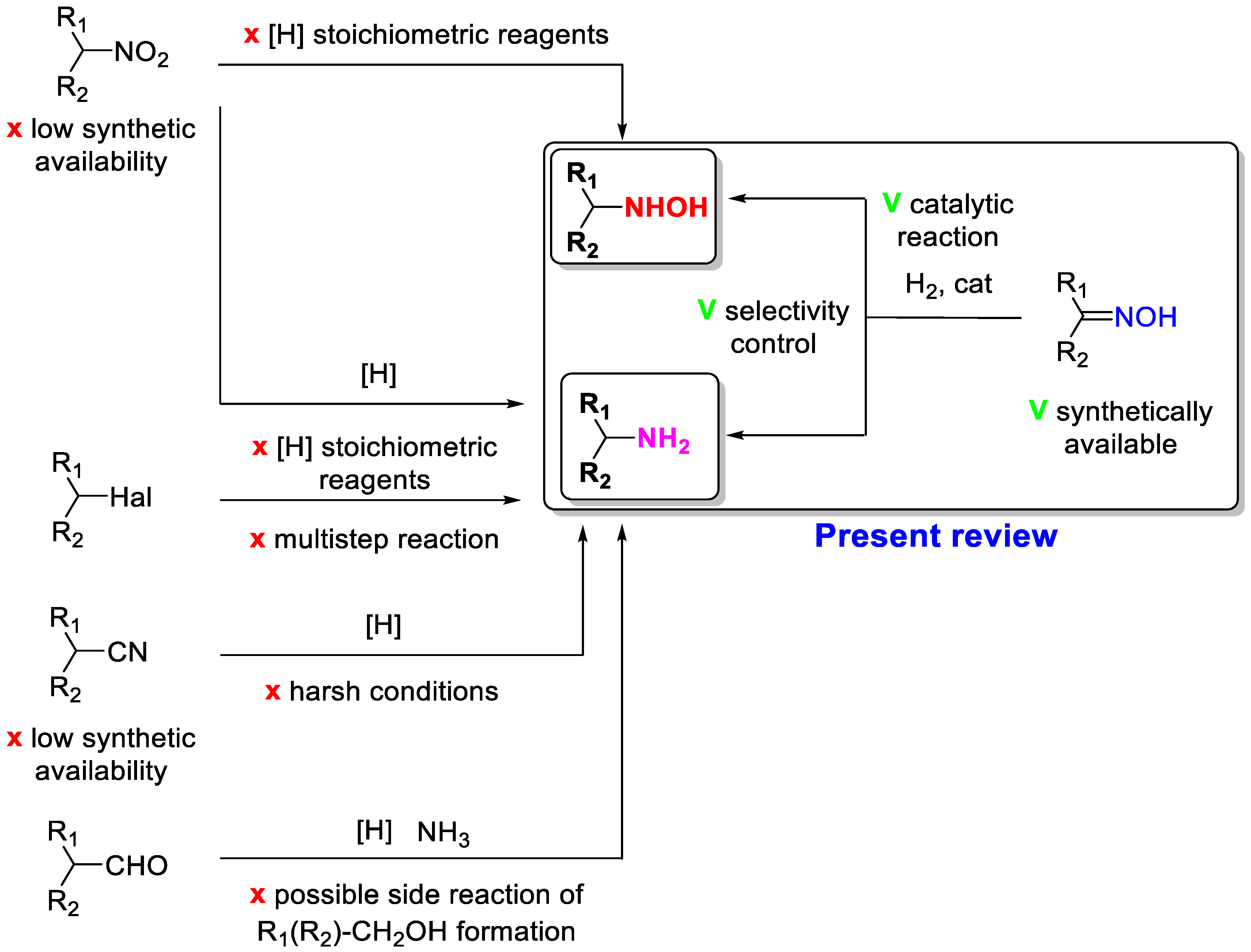
On the other hand, heterogeneous catalytic hydrogenation of oximes is the simplest way to obtain amines or hydroxylamines
[3]. The main advantages of this methodology are the use of easily available oximes and a benign reducing agent—hydrogen—as well as the possibility to control the selectivity to amine or hydroxylamine formation (
Scheme 1). Oximes are obtained by reacting a carbonyl compound with hydroxylamine hydrochloride (
Scheme 1). Therefore, this reaction allows for the synthesis of a wide range of oximes with a different structure, the catalytic hydrogenation of which leads to a variety of amines or hydroxylamines. This is one of the most applied steps in drug synthesis.
2. Hydrogenation of Ketoximes to Amines
2.1. Noble Metal Catalysts
The hydrogenation of ketoximes over Pt- or Pd-based catalysts is actively applied in pharmaceutical production, which is usually based on multistep organic synthesis processes, as an efficient tool to convert ketones to the corresponding amine products through the oxime formation step. In the very first work on selective oxime hydrogenation reported by Vavon et al., PtO
2 (Adam’s catalyst) was shown to be an active and selective catalyst for the synthesis of N-hydroxy/alkoxy derivatives of amphetamines from aryl-acetone oximes at room temperature and a hydrogen pressure of 3 atm in the presence of an equivalent amount of HCl
[4]. PtO
2 was applied in the hydrogenation of terpenoid oximes, such as carvone oxime, fenchone oxime, and camphor oxime
[5]. Recently, Pt supported on TiO
2, ZrO
2, Al
2O
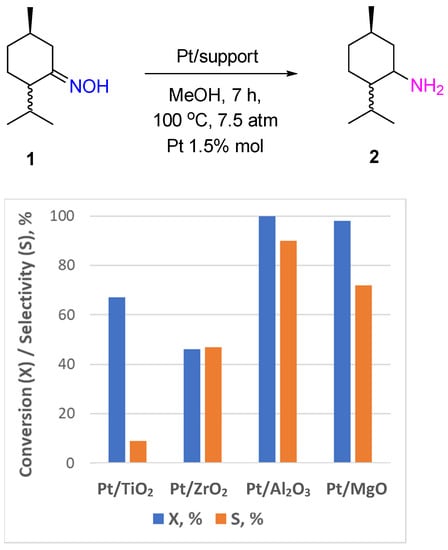
3, and MgO was also used for hydrogenation of monoterpenoid oximes (menthone oxime
1 and carvone oxime
3,
Scheme 2) to the corresponding amines
[6]. Menthylamine
2 can be obtained with a 90% yield over Pt/Al
2O
3 catalysts after 7 h of the reaction at 100 °C and a hydrogen pressure of 7.5 atm in MeOH (
Figure 1). The Pt supported on TiO
2, ZrO
2, and MgO was less selective, and the process led mostly to ketone formation. The same trend was observed in carvone oxime hydrogenation: Pt/TiO
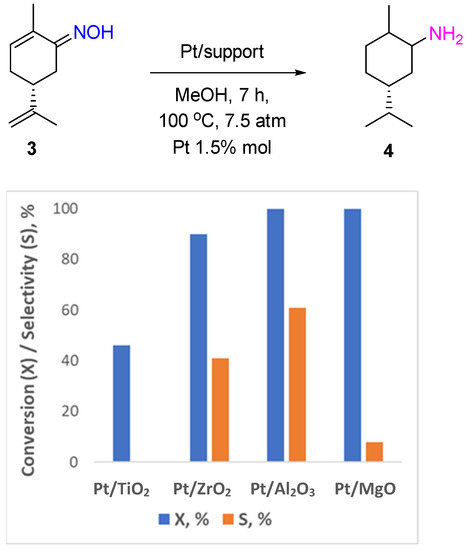
2 and Pt/MgO catalyzed the deoximation reaction that proceeded most likely via the hydrolysis of the substrate molecule with the participation of hydroxyl groups of the support. However, all reducible groups in the carvone molecule were hydrogenated over Pt/Al
2O
3 with the formation of 5-isopropyl-2-methylcyclohexanamine
4 with a yield of up to 60% (
Figure 2).
Figure 1. Hydrogenation of menthone oxime over Pt-supported catalysts.
Figure 2. Hydrogenation of carvone oxime over Pt-supported catalysts.
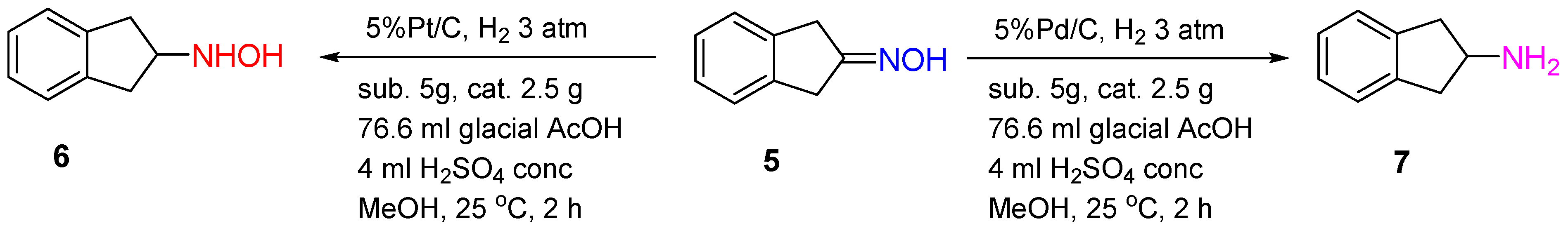
Scheme 2. Hydrogenation of 2-indanone oxime over Pt/C or Pd/C catalysts.
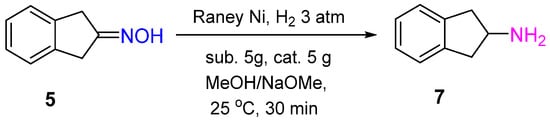
The Pd/C catalyst was shown to provide 2-indanone oxime
5 hydrogenation to 2-aminoindane
7 with a yield of 94% in the presence of glacial AcOH and H
2SO
4 (conc.) after 2 h of the reaction at 20–25 °C and 3 atm of the hydrogen pressure. Interestingly, using 5%Pt/C under the same conditions led to hydroxylamine
6 formation with a yield of 54% (
Scheme 2)
[7].
The differences in the mechanism of oxime hydrogenation over Pd and Pt catalysts can explain the differences in the hydrogenation products. The studies of Rosen and Green showed that Pd catalyzed N-O bond reduction, while Pt catalyzed the reduction of a C=N bond in the protonated 2-indanone oxime.
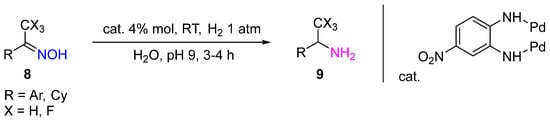
Pd-containing complexes can also be used as heterogeneous catalysts. Thus, the Pd complex with a 4-nitrobenzene-1,2-diamine ligand provided selective hydrogenation of oximes
8 in water under H
2 atmospheric pressure and RT with a quantitative yield of corresponding amines (
Scheme 3)
[8]. The catalyst can be easily isolated from the reaction product and used again without additional treatment.
Scheme 3. Hydrogenation of ketoximes over Pd complex with a 4-nitrobenzene-1,2-diamine ligand.
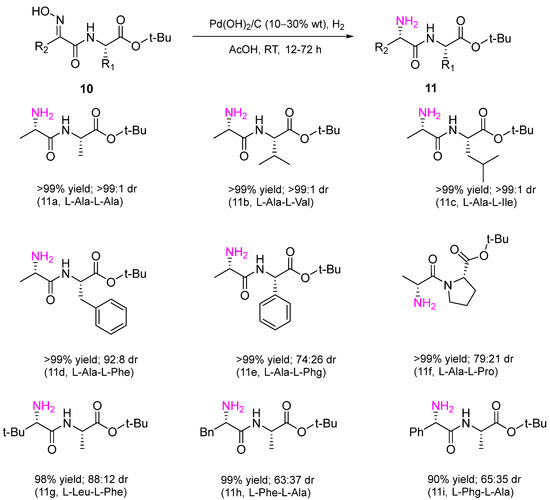
In the recent work, a heterogeneous Pd catalyst, Pd(OH)
2/C, was used in diastereoselective hydrogenation of amides
10 to obtain dipeptides
11 of different structures with a new chiral center with high diastereoselectivity in excellent yields (
Scheme 4)
[9].
Scheme 4. Diastereomeric hydrogenation of amides bearing an oxime group to dipeptides over a Pd(OH)2/C catalyst; dr–diastereomeric ratio.
The reaction was performed in AcOH at RT and H2 atmospheric pressure. The method has significant synthetic value. Due to the high specificity of peptides compared to small molecule drugs, they are considered promising pharmaceuticals for the treatment of cancer and tuberculosis and for antimicrobial therapy.
Enantioselective hydrogenation of oximes was also reported on Pd and Pt catalysts
[10][11][12], but enantiomeric excess (
ee) was not enough for the practical implementation of this approach in pharmaceutical synthesis in contrast to homogeneous hydrogenation of oximes by metal complexes with chiral ligands
[13][14]. For instance, 5%Pd/Al
2O
3 modified with natural chiral amino alcohol (1R,2S)-(−)-Ephedrine was applied for enantioselective hydrogenation of pyruvic acid oxime to alanine at 30 °C in ethanol under H
2 pressure of 10 atm
[10]. The yield of target alanine did not exceed 15%, with
ee of
S-alanine being only 26%.
2.2. Non-Noble-Metal Catalysts
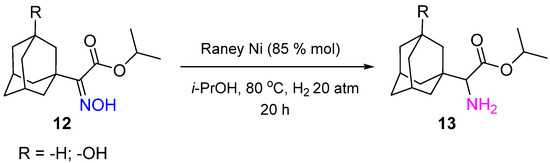
Non-noble metal catalysts based on Co and Ni are effectively applied in oxime hydrogenation. Raney Ni was used to synthesize 1-adamantyl-glycine derivatives
13, which are known to possess high antiviral activity to influenza virus A
[15]. The synthesis was realized by hydrogenation of corresponding oxime
12 at 80 °C and H
2 pressure of 20 atm in 20 h (
Scheme 5).
Scheme 5. Synthesis of 1-adamantyl-glycine derivatives by hydrogenation of a corresponding oxime over Raney Ni.
Under mild reaction conditions, Raney Ni showed high catalytic activity in the hydrogenation of 2-indanone oxime
5 with the yield of corresponding amine
7 of 91%. In contrast to Pt and Pd catalysts, to obtain the high yield of amine over Raney Ni, basic conditions were required (
Scheme 6)
[7].
Scheme 6. Hydrogenation of 2-indanone oxime to 2-aminoindan over Raney Ni.
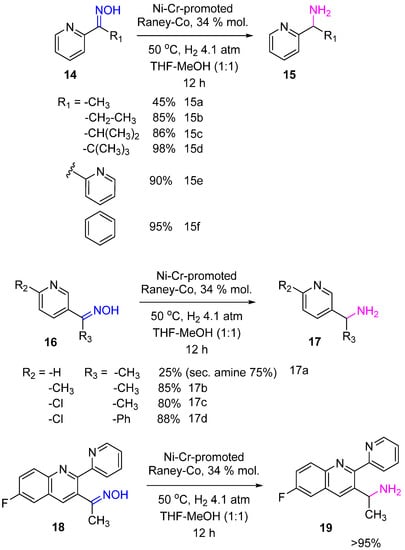
Baucom et al. used Ni–Cr-promoted Raney-Co for the hydrogenation of ketoximes as a synthetic step in transforming heteroaromatic ketones to the corresponding amines, an advanced intermediate of a kinase inhibitor
[11]. Ketoximes
14,
16,
18 were hydrogenated to the primary amines
15,
17,
19 with yields of 85–99%, and only for less sterically hindered substrates (14a, 16a), the competitive formation of secondary amines was a problem (
Scheme 7).
Scheme 7. Hydrogenation of heteroaromatic ketoximes to amines over Ni–Cr-promoted Raney-Co.
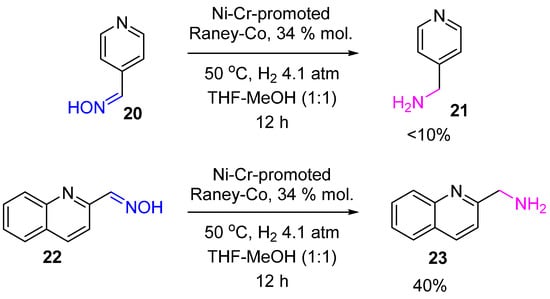
3. Hydrogenation of Aldoximes to Amines
Reduction of aldoximes with H
2 over heterogeneous catalysts is usually accompanied by a side reaction of secondary amine formation due to the high steric accessibility of the oxime carbon atom. Thus, hydrogenation of heteroaromatic aldoximes
20,
22 on the above-mentioned Ni–Cr-promoted Raney-Co proceeded unselectively with the yield of corresponding primary amines
21,
23 not exceeding 40% (
Scheme 8)
[16].
Scheme 8. Hydrogenation of heteroaromatic aldoximes to amines over Ni–Cr-promoted Raney-Co.
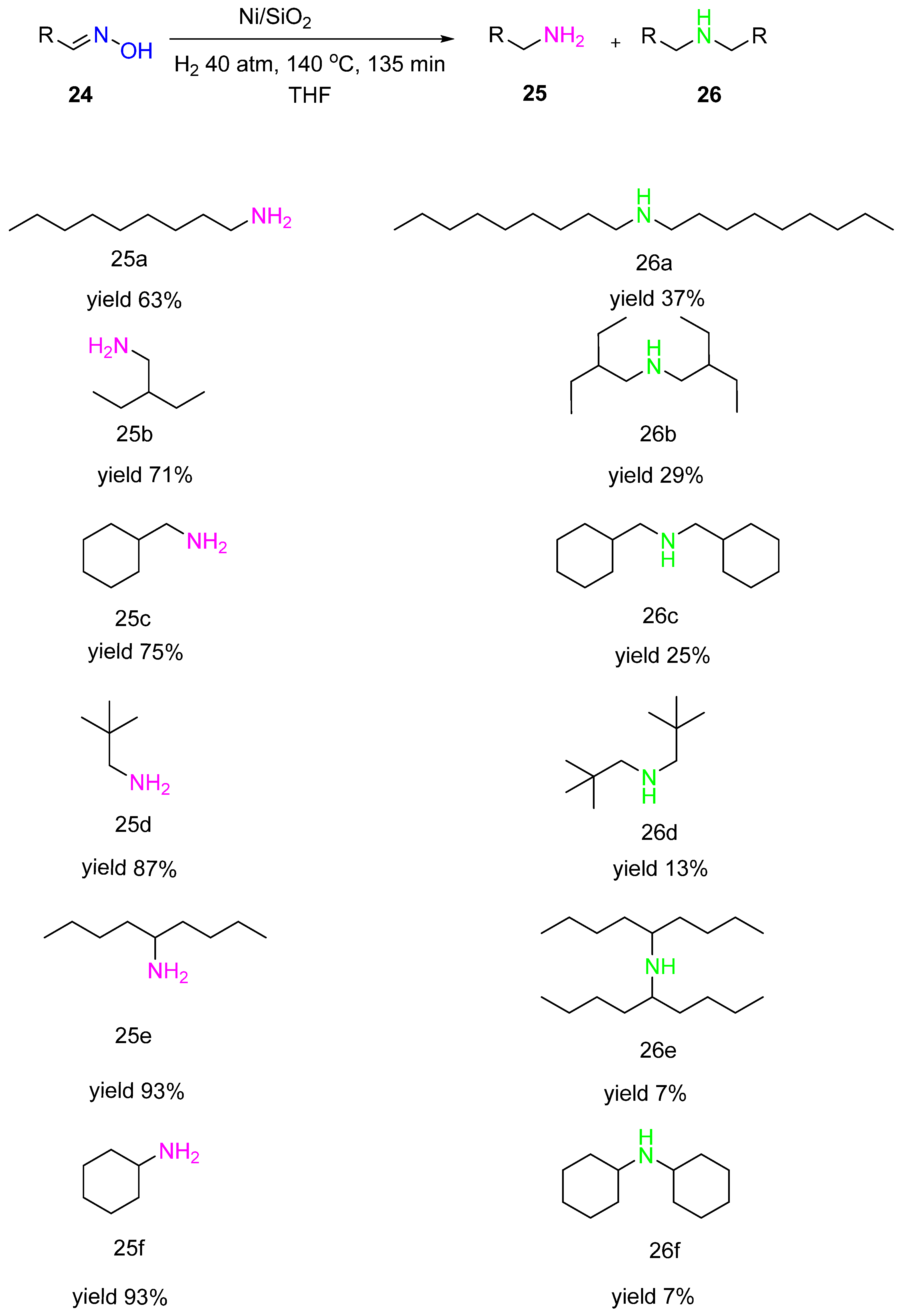
Gebauer-Henke with co-authors showed the same selectivity trend when studying hydrogenation of alkyl aldoximes
24 over Ni/SiO
2 catalysts (Ni 46.6% wt., Cr 9.9% wt.) (
Scheme 9)
[17]. It was also observed that the selectivity to the primary amine
25 increased with the increasing steric demand of the substituent. In contrast to aldoximes, hydrogenation of ketoximes proceeded with a considerably higher selectivity to the primary amines. This fact proves that steric hindrance at the oxime carbon atom is one of the critical factors for the high selectivity of primary amines in hydrogenation.
Scheme 9. Hydrogenation of aliphatic aldoximes to amines over Ni/SiO2.
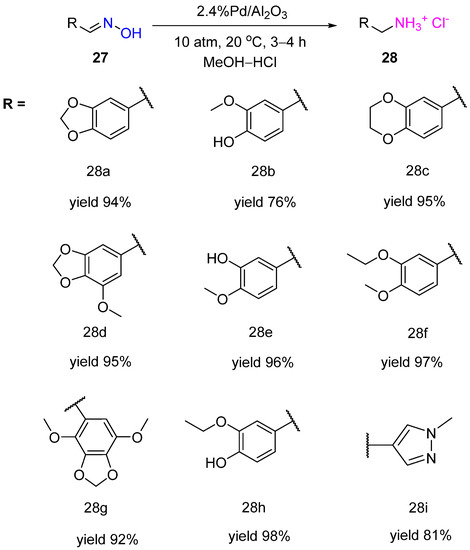
Nothwithstanding, the selective hydrogenation of aldoximes can be afforded in acidic media. Ignatov et al. showed that Pd supported on high-porosity foamed ceramic material containing 6% γ-Al
2O
3 appeared to be an active, selective, and recyclable catalyst for hydrogenation of benzaldoximes
27 to the corresponding primary amines
28 at RT and hydrogen pressure of 20 atm in a methanolic solution of HCl (3.6%
v/
v HCl) (
Scheme 10)
[18]. The obtained benzylamine derivatives can be used as synthons for the preparation of anticancer drugs and psychotropic substances for the treatment of mental disorders. The deposition of Pd on alumina-containing porous ceramic material enabled more than 20 recycles of the obtained catalyst.
Scheme 10. Hydrogenation of aromatic aldoximes to amines over Pd/Al2O3.
4. Hydrogenation of Oximes to Hydroxylamines
Hydroxylamines are valuable compounds for both pharmaceutical and agrochemical synthesis. The most prominent way to obtain hydroxylamines is oxime hydrogenation. However, it is a challenging task because hydrogenation of a weak N-O bond is more thermodynamically favorable than hydrogenation of a C=N bond. Only a few examples exist in the literature on oxime hydrogenation to hydroxylamines
[3].
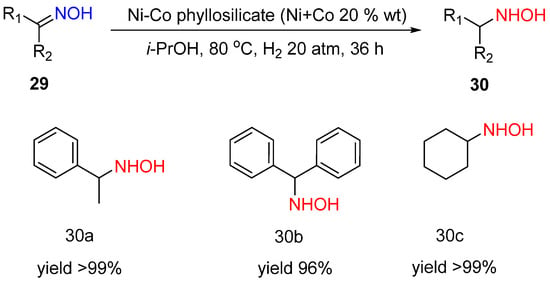
The Ni-Co phyllosilicate (Ni:Co = 1:1) was shown to provide selective hydrogenation of oximes
29 to hydroxylamines
30 with the yields 96–99% at 80 °C and an H
2 pressure of 20 atm in 36 h (
Scheme 11)
[19]. The reaction can be performed without acid additives. At the same time, the catalyst is not stable, and it loses the activity from cycle to cycle because of the Co surface oxidation and poisoning of the active sites by products. However, the reason for the observed unusual selectivity in oxime hydrogenation to hydroxylamine was not discussed.
Scheme 11. Hydrogenation of ketoximes to hydroxylamines over Ni-Co phyllosilicate.
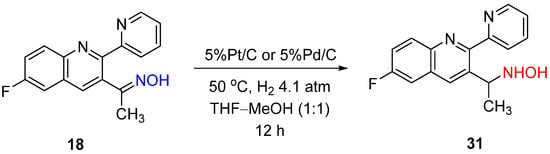
As researchers mentioned above, Rosen and Green noticed the change in the selectivity from amine to hydroxylamine when Pt/C was used instead of Pd/C for 2-indanone oxime hydrogenation (
Scheme 2)
[7]. In contrast, Baucom et al. reported that Pd/C or Pt/C catalysts led to the formation of hydroxylamine in the case of hydrogenation of ketoxime
18 (
Scheme 12)
[16].
Scheme 12. Example of ketoxime hydrogenation to hydroxylamine over Pd/C or Pt/C catalysts.
The last example is the already mentioned work of Vavon et al., where it was shown that hydrogenation of aryl-acetone oximes on PtO
2 in the presence of an equimolar amount of HCl led to N-hydroxy/alkoxy derivatives of amphetamines
[4]. The variety of hydroxylamines obtained by this method and its limitations were recently reviewed and thoroughly discussed by Mas-Roselló and Cramer
[3].