Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Plant-derived flavonoids are considered natural nontoxic chemo-preventers and have been widely studied for cancer treatment in recent decades. Mostly all flavonoid compounds show significant anti-inflammatory, anticancer and antioxidant properties. Kaempferol (Kmp) is a well-studied compound and exhibits remarkable anticancer and antioxidant potential. Kmp can regulate various cancer-related processes and activities such as cell cycle, oxidative stress, apoptosis, proliferation, metastasis, and angiogenesis.
- kaempferol
- flavonoids
- cell signalling pathways
1. Inflammation
Inflammation is a biologically complex protective body’s reaction rising due to dangerous stimuli and damaged cells. Many diseases are characterized by inflammation including allergy, transplant rejection, preperfusion injury, hepatitis, glomerulonephritis, asthma, autoimmune disorders, celiac disease, intestinal inflammation and cancer [41]. Hence, inflammation is a biologically, self-protecting body’s reaction in times of problem which eliminates injured and damaged cells and starts the healing process [42]. It has been suggested that chronic inflammation is linked with the progression of several disorders such as neurodegeneration, cancer, and arthritis [43,44,45]. Kmp has been recognised as an effective inhibitor of pro-inflammatory molecules including vascular cell adhesion protein 1, prostaglandin-endoperoxide synthase (PTGS) and inducible nitric oxide synthase (NOSII) [46,47]. Anti-inflammatory effects of Kmp are mostly facilitated by downregulation of numerous sequence-specific DNA-binding factors like STAT, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which have the capability to encourage the pro-inflammatory cytokines activation [48]. A study analysed the anti-inflammatory property of Kmp in hepatic cell lines and found that Kmp reduced the PTGS, NOSII, C-reactive protein (CRP) expression by altering NF-κB signalling pathway [49]. The ability of Kmp to deal with inflammation is one of its critical and considerable features in cancer prevention (Table 1). When lipopolysaccharide-induced macrophages treated with Kmp, it resulted into downregulation of PTGS, NOSII and tumor necrosis factor-alpha (TNF-alpha) at translational- and transcriptional- levels through inhibiting sequence-specific DNA-binding factors such as Activator protein 1 (AP1) and NF-κB [50,51]. Furthermore, protein-kinase signalling cascades mechanism directed by interleukin-1 receptor-associated kinase (IRAK)-1, -4, Syk and Src which are generally take part in AP1 and NF-κB factors activation and could prevented by Kmp [52]. In diseases like Crohn’s disease or rheumatoid arthritis, uncontrolled inflammation can cause immune system arrest where immune system harms normal healthy cells. Chronic inflammation is associated with a susceptibility in development of cancer [53]. Stomach ulcer is linked with an increasing risk of peptic cancer and mesothelioma can be tracked back to irritation caused by asbestos. It has been reported that flavonoid (particularly Kmp) rich diet is correlated with decreased level of serum interleukin-6 which is an inflammatory cytokine [54]. In aldosterone induced human umbilical-vein endothelial cell (HUVEC), Kmp has been reported to downregulate the expression of ROS-dependent cytokines such as osteopontin which activates and stimulates NF-κB and p38-mitogen-activated protein kinases (p38-MAPK) signalling [47]. Hence, studies are recommending Kmp as a promising anti-inflammatory drug and it can be proposed for in-vivo trials.
2. Reactive Oxygen Species (ROS)
Metabolic pathways generate ROS in the body which are key resource of destructive oxidative stress [55,56]. Though humans have antioxidant enzymes as defence mechanisms which continuously neutralises ROS, but high ROS concentration causes infections, senescence, cerebrovascular accident, autoimmune disorders, cardiovascular arteriosclerosis, oxygen poisoning, Parkinson’s disorder and becomes lethal [55,57]. Studies suggested that flavonoids can be efficient secondary-metabolites against oxidative stress-related diseases [58]. Kmp increases the anti-oxidant enzymes expressions at high concertation and at low-concentration it scavenges hydroxyl (OH) radical and peroxonitrite radical [30]. The antioxidant property of Kmp is linked with its up regulatory effects on antioxidant-response element- (ARE) meditative anti-oxidative enzymes like superoxide dismutase, catalase and haem oxygenase in control of Nuclear factor erythroid 2-related factor 2 signalling pathway [59]. Kmp can be used in prevention of susceptibility of oxidation of low density lipoproteins (LDL) and aggregation of platelets [60]. Both Wahab et al. 2014 and Choe et al. 2012 studied antioxidant property of Kmp by extracting and purifying Kmp from Senna alata beans and Rhodiola sachalinensis roots respectively [61,62]. It has been observed that Kmp reduced the thiobarbituric-acid reactive substances and red blood corpuscles lysates and upregulated the level of enzymatic antioxidants such as superoxide dismutase, glutathione perxidases (GSHPx) and catalase when 1,2-dimethylehydrazine (DMH)-induced-colon cancer male Wistar-rats treated with Kmp [63]. Similarly, researchers studied hepatoprotective effects of Kmp by increasing in carbon tetrachloride (CCl4)-induced liver damage in rodents [64,65]. Kmp reduces the level of reactive oxygen and increases the survival of cell in oxidatively stressed HT 22 neuronal cells and reduces oxidative DNA-damage in isolated human lymphocytes [66].
3. Angiogenesis
Cancerous cells also need nutrients and oxygen to survive provided with networks of capillaries. Angiogenesis is linked with repair of damaged cells and reproductive development via formation of new capillaries which is mediated by growth molecules, endostatins, adhesion molecules etc. [67]. Main mediator in angiogenesis is VEGF and formation of new capillaries aimed to meet increasing requirements of the tumor [68]. Current studies have demonstrated the efficiency of Kmp in reducing angiogenesis of cancer in in-vitro and in-vivo by preventing secretion of VEGF in human cancerous cells [69,70]. A study reported that Kmp prevented VEGF secretion in MDA-MB-231 cancerous cells and decreased the concentration of VEGF-mRNA among ovarian cancerous cell lines [71]. Level of VEGF proteins was significantly influenced by Kmp, indicating action-mechanism involved in translation [67]. Kmp inhibits angiogenesis and expression of VEGF via ERK-NFkappaB-cMyc-p21 pathways [70]. Administered Kmp inhibited expression of NF-κB, c-Myc and phosphorylation of ERK and reduction of these encourages expression of p21 which antagonizes the release of VEGF [68]. Moreover, Kmp also affected regulators of VEGF. Kmp reduces the level of hypoxia inducible factor (HIF)-1 and inhibits phosphorylation of AKT signalling pathway and it blocks signalling mechanisms which involves in enhanced VEGF secretion [67]. Kmp also inhibits activity of estrogen related receptor alpha (ESRRA) by reducing its mRNA level. ESRRA is linked with oestrogen-activity and considered as a cancer promoter. Kmp is an opponent of VEGF and attacks production of VEGF from every path (Figure 3) [23].
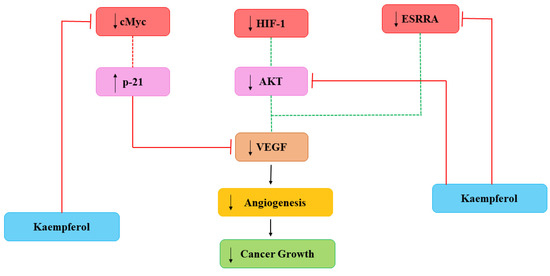
Figure 3. Kaempferol effects on Angiogenesis. HIF-1: hypoxia inducible factor-1; VEGF: vascular endothelial growth factor; ESRRA: estrogen related receptor alpha. Dotted lines signify earlier processes that have decreased due to Kmp [23].
4. Signal Transduction
Numerous interleukin-6 related signalling pathways have been linked and found with increased migration, invasion, and proliferation of several tumor cells. Interleukin-6 binds with interleukin-6 specific binding receptor-α and activates the dimerization of signal-transducer receptor called glycoprotein 130 and causes its phosphorylation, followed by Janus tyrosin kinase (JAK) activation [72]. These incidents cause the activation of several signal-transduction pathways, like signal-transducer and activator of transcription (STAT), PI-3 kinase signalling pathways [72]. In all these pathways, STAT3 signalling pathway is the mostly analysed/studied cytokine-signalling pathway [73,74]. STAT3 is a member of STAT-family of transcription-factors and plays an important role in cancer related inflammation. STAT3 is often de-regulated in several kinds of cancer and function as an onco-gene in tumorigenesis [75]. STAT3 activation causes expression of down-stream genes which regulate main cell responses (includes survival of cancerous cell, cell invasion and proliferation) like BCL2, cyclin-D1 and MMP-2 [76]. STAT3 plays important role in tumorigenesis and in progression of cancer which allow STAT3 to arise as a promising molecule target in the treatment of cancer. Basu et al. (2020) observed that at high concentration, Kmp prevented interleukin-6 induced-phosphorylation of STAT3 [77]. A study conducted by Yang et al. (2019) concluded that Kmp inhibits STAT3 signalling pathway [78].
Phosphatidylinositide-3-Kinase (PI3K) is an important signal-transducing enzyme which regulates cell differentiation, survival, angiogenesis, proliferation, and apoptosis [79,80]. It is vital for AKT activation and has an important role in pathological as well as physiological signalling processes. Due to the repeated activation of PI3K-AKT mechanism in cancer, it is a key drug target [81,82,83,84,85]. PI3K is a lipid-kinase which causes phosphorylation PIP2–PIP3 and it is the PDK and AKT activation site. Family of PI3K has three different classes viz, class I, II and III and these classes are different in distribution of tissue, in function, preference of substrate, activation pathway and structure [86,87,88]. PI3K-dependent AKT activation results into multi step method which involve both phosphorylation as well as translocation [89]. Activation of AKT includes the phosphorylation of two residues: serine 473 (Ser473) at carboxy-terminal and threonine 308 (Thr308) on activation loop. Ser473 is phosphorylated by PDK2 while PDK1 phosphorylated by Thr308 [90,91]. PDK1 is an important kinase needed for normal development in mammals [92]. AKT has three isoforms: AKT-1, AKT-2 and AKT-3 based on their different biological activities and distribution of tissue. AKT-1 plays a vital role in angiogenesis and cell survival regulation [86,93,94]. PI3K activation is counter-production to apoptotic pathway and due to this, several drugs related to cancer treatment concentrate on inhibition of this pathway. Chin et al. (2018) reported that Kmp in dosage-dependent manner significantly reduced the mTOR and AKT phosphorylation and level of PI3K protein [95]. Another study reported that Kmp repressed the growth of colorectal cancerous cells by preventing the activation of PI3K-AKT signalling pathways [96].
Some studies reported the apoptosis inducing properties of Kmp which can be partly accredited to its impacts on pathway of MAPK. In A-549 and MCF-7 cell lines, initiation of MAPK pathway is a key factor in Kmp-induced apoptosis. Moreover, Kmp-mediated activation of MAPK can block DNA damage which leads to transformation of cell. Kmp presence increases the expression of haemoxygenase-1 gene (HO-1), which triggers the rise in antioxidant ability of cells [97]. Treatment of Kmp significantly increased the viability of cells in response to oxidative stress, which involves unstable free radicals susceptible to damage DNA. Thus, Kmp-induced MAPK induction defends healthy cells from converting into cancer cells. RSK2 is a major suppressor of apoptosis, it downregulates the BAD, a protein which promotes apoptosis and upregulates the Bcl-2 level [98]. It has been observed that Kmp directly binds to RSK2 protein particularly at lysine-100 (Lys) and valine-82 (Val) positions, which plays an important role in RSK2 functioning [99]. Thus, Kmp paralyzes the RSK2. Obviously, treatment dropped Bcl level and increase concentration of tumor suppressor protein such as p53 and BAD [98]. Moreover, Kmp has also been reported to interrupt activity of Src-kinase [100]. MAPK is activated by Src in pro growth situation, which activates the COX-2 protein, and occurrence of COX-2 is a cautionary marker for skin tumor [101]. MAPK-ERK pathway is modified at various crucial sites by Kmp (Figure 4).
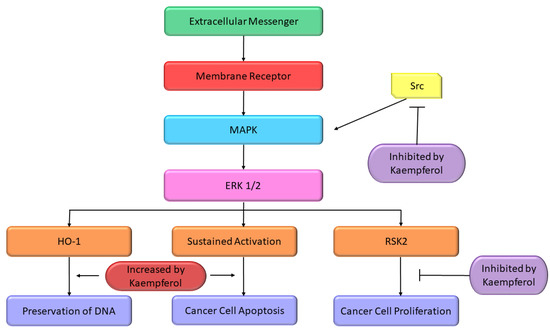
Figure 4. Effect of Kmp on MAPK pathway [26].
Hence, Kmp affects STAT3, PI3K signalling and MAPK pathway and exhibits significant potential in manipulation of cell-signalling pathways in apoptosis initiation and leaves normal cells alone.
5. Cell Cycle
A cell cycle is repeating series of events which involves copying of contents of cell and following division. Cells are continuously subject to DNA mutation that is harmful for cells but hardly results in cells production which can avoid the normal restrictions and flourish as pathologic tumors [102]. The development and progression of cancer is often associated with disruption or dysregulation of normal cell-cycle progression. Cells react to damage in DNA by stopping cell cycle progress and / or by enduring apoptosis [102].
Several flavonoids and natural chemo preventers including Kmp have been observed to precisely regulate numerous proteins which are involved in cellular homeostasis and cell cycle, whose de-regulation may play a role in carcinogenesis [103,104]. The ability of Kmp to induce cell cycle arrest have been observed in several cell cycles like in a study conducted by Gao et al. (2018) found that Kmp treatment induces G2-M phase cell cycle arrest through checkpoint kinase2 (CHK2) in ovarian cancerous cells [105] and Xu et al. (2008) in their study observed that Kmp induces G2-M phase cell cycle arrest in cervical cancerous cells [106], it has been reported that Kmp therapy can lead to G0-G1 cell cycle arrest in human esophageal squamous carcinoma Eca-109 cells [107]. Kmp treatment increased the level of p53 in MDA-MB-453 breast cancerous cells [108]. Furthermore, gene c-Myc is usually overexpressed in cancerous cells which leads to uncontrolled cell proliferation [109]. Studies showed that enhanced c-Myc level antagonized mRNA concentration of CDKN1A [110], administration of Kmp in combination with cisplatin reduces mRNA concentration of c-Myc and increases mRNA concentration of CDKN1A in ovarian cancerous cells. Cisplatin alone cannot kill cancerous cells, however, in combination with Kmp, they initiate apoptotic pathway via hindering c-Myc expressions in cancerous cells [111]. p53 is famous tumor suppressor protein generally indicated as ‘guardian of genome’ [111]. Repairing of damaged DNA is generally regulated by p53 [111]. Luo et al. (2011) observed that Kmp prevented phosphorylation of AKT signalling but upregulated the p53 expression and induced apoptosis in ovarian cancerous cells (Figure 5) [98]. Kmp is a useful flavonoid with genuine ability in disrupting growth of cancer and deserves more study into its impact on the cell cycle. A versatile chemoprophylactic molecule, kmp appears to play a role in each part of growth of cancer. Indeed, there persist a host of kmp-sensitive genes awaiting to be studied [112]. Kmp can efficiently prevent the proliferation and activation of mice T-lymphocytes in response to ConA, and can arrest cell cyle at G2/M and S phases [113].
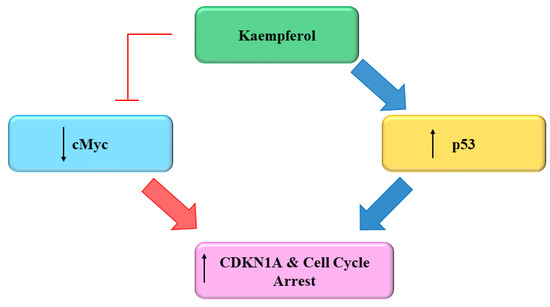
Figure 5. Effect of Kaempferol on the Cell Cycle [112].
6. Remodeling Tumor Metabolism
Metabolic remodeling is a phenomenon of the occurrence and development of tumors. It provides energy and material to the cells for survival and proliferation and prepares cells to survive in the harsh microenvironment [114]. Kaempferol inhibit both growth and migration of glioma cells, even when kaempferol was loaded to mucoadhesive nanoemulsion (KPF-MNE) or kaempferol-loaded nanoemulsion (KPF-NE) [27].
Table 1. Major mechanism of action of Kaempferol (Kmp) in cancer management.
| Major Mechanism | Outcome of the Study | Refs |
|---|---|---|
| Inflammation | Kmp has been recognised as an effective inhibitor of pro-inflammatory molecules including vascular cell adhesion protein 1, prostaglandin-endoperoxide synthase (PTGS) and inducible nitric oxide synthase (NOSII) | [46,47] |
| Inflammation | Anti-inflammatory effects of Kmp are mostly facilitated by downregulation of numerous sequence-specific DNA-binding factors like STAT, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) which have the capability to encourage the pro-inflammatory cytokines activation | [48] |
| Reactive Oxygen Species (ROS) | The ant-oxidant property of Kmp is linked with its up regulatory effects on antioxidant-response element- (ARE) mediative anti-oxidative enzymes like superoxide dismutase, catalase, and haem oxygenase in control of nuclear factor erythroid 2-related factor 2 signalling pathway | [59] |
| Reactive Oxygen Species (ROS) | Kmp reduced the thiobarbituric-acid reactive substances and red blood corpuscles lysates and upregulated the level of enzymatic antioxidants such as superoxide dismutase, glutathione perxidases (GSHPx) and catalase when 1,2-dimethylehydrazine (DMH)-induced-colon cancer male Wistar-rats treated with Kmp | [63] |
| Angiogenesis | Kmp prevented VEGF secretion in MDA-MB-231 cancerous cells and decreased the concentration of VEGF-mRNA among ovarian cancerous cell lines | [71] |
| Angiogenesis | Administered Kmp inhibited expression of NF-κB, c-Myc and phosphorylation of ERK and reduction of these encourages expression of p21 which antagonizes the release of VEGF | [70,71] |
| Signal transducer and activator of transcription 3 (STAT3) | At high concentration, Kmp prevented interleukin-6 induced-phosphorylation of STAT3 | [77] |
| Phosphatidylinositide-3-kinases (PI3K)-AKT pathways (PI3K-AKT) | Kmp repressed the growth of colorectal cancerous cells by preventing the activation of PI3K-AKT signalling pathways | [96] |
| Cell cycle | Kmp treatment induces G2-M phase cell cycle arrest through checkpoint kinase2 (CHK2) in ovarian cancerous cells or it has been shown that Kmp therapy can lead to G0-G1 cell cycle arrest in human esophageal squamous carcinoma Eca-109 cells | [105,106,107] |
| Cell cycle | Administration of Kmp in combination with cisplatin reduces mRNA concentration of c-Myc and increases mRNA concentration of CDKN1A in ovarian cancerous cells | [111] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules27248864
This entry is offline, you can click here to edit this entry!
