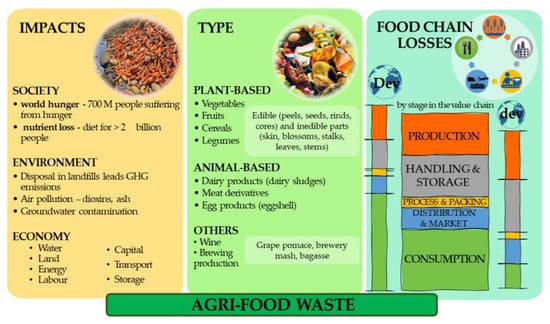
In the upcoming years, the world will face societal challenges arising, in particular, from the impact of climate change and the inefficient use of natural resources, in addition to an exponential growth of the world population, which according to the United Nations (UN) estimations will be 9.8 billion in 2050. This increasing trend requires optimized management of natural resources with the use of value-added waste and a significant reduction in food loss and food waste. Moreover, the recent pandemic situation, COVID-19, has contributed indisputably. Along with the agri-food supply chain, several amounts of waste or by-products are generated.
- agri-food waste
- valorisation
- food loss
- food waste
1. Introduction
2. Extraction Techniques for Bioactive Recovery from Agri-Food Wastes
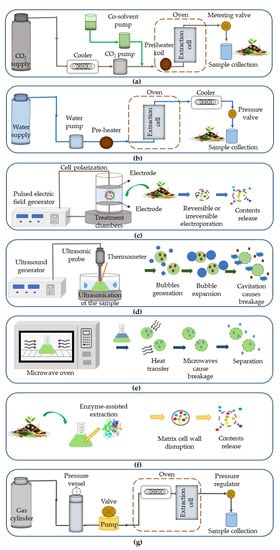
2.1. Supercritical Fluid Extraction
| Agri-Food Waste (Amount) | Targets | Extraction Conditions | Extraction Efficiency | Ref. |
|---|---|---|---|---|
| Supercritical fluid extraction | ||||
| Agave salmiana bagasse (10 g) |
Antioxidants and saponins | CO2, 60 °C, 300 bar, 10 % v/v ethanol, 1.7 g/min, 60 min | Increase in the antioxidant activity in the US-assisted extraction from 11.54 ± 0.06 to 17.61 ± 0.75 μmol of Trolox equivalents/g | [12] |
| Avocado peel and seeds (-) |
Catechin, quercetin | CO2, 80 °C, 250 bar, ethanol ratio of 1:1.5 S:L, 30 min | Integral biorefineries of avocado seed and peel allow profit margins of 47% and 43%, respectively | [18] |
| Capsicum annuum waste (20 g) | Phenolics, flavonoids, fatty acids, and carotenoids | CO2, 40 and 60 °C, 200 and 250 bar, with and without ethanol, 3 g/min, 30 min | Yield 9.38–10.08%, phenols (12.30–23.94 mg/g), flavonoids (0.6–1.52 mg/g), and carotenoids (0.27–2.01 mg/g) | [13] |
| Carrot peels (50 g) |
Carotenoid | CO2, 59 °C, 349 bar, 15% v/v ethanol, 15 g/min, 80 min | Carotenoid recovery was (86.1%) with 97 % purity | [10] |
| Grape seeds (17 g) |
Triacylglycerols | CO2, 40–60 °C, up 400 bar, 1.8–2.8 g/min | Oil yield in the range of 12.0–12.7%, as compared to 12.3% obtained by a conventional n-hexane extraction | [19] |
| Mandarin peel (100 g) |
Limonene, hesperidin | CO2, 130–220 °C, 100–300 bar, 33 g/min, 90 min | Limonene (13.16 and 30.65% at 100 and 300 bar), hesperidin (0.16–15.07 mg/g) | [20] |
| Mango peel (5 g) |
Carotenoids | CO2, 60 °C, 250 bar, 15% w/w ethanol, 6.7 g/min, 180 min | Carotenoids (1.9 mg all-trans-β-carotene equivalent/g dried mango peel) | [11] |
| Melon seeds (5 g) |
Phytosterols | CO2, 33 °C, 200 bar, 11 g/min, 3 h | β-sitosterol (304 mg/kg) and stigmasterol (121 mg/kg) | [21] |
| Pomegranate seed (100 g) |
Seed oil | CO2, 60 °C, 320 bar 133 g/min, 180 min | Oil (85.4% of punicic acid) | [17] |
| Subcritical water extraction | ||||
| Citrus peel (3 g) |
Hesperidin, narirutin | Water, 110–190 °C, 10 MPa, 3–15 min | Hesperidin (6.96 mg/g peel dw), narirutin (8.76 mg/g peel dw) | [22] |
| Grape pomace (50–60 g) | Phenolic compounds | Water, 130–190 °C, 100 bar, 10 mL/min | 29 g of phenolic compounds (p-hydroxyphenyl, guaiacyl, and syringyl)/100 g extract | [23] |
| Kiwifruit peel (2% S:S ratio) | Phenolic compounds | Water, 160 °C, S:S ratio (2%), pH 2, 20 min | TPC (51.2 mg GAE/g dw), TFC (22.5 mg QE/g dw) | [24] |
| Onion peel (2 wt % onion skins into 600 mL of H2O) |
Phenolic compounds | Water, 170–230 °C, 30 bar, 400 rpm/30 min | 63–75 mg GAE/g, 23–26 QE/g | [16] |
| Onion skin (4 g) |
Phenolic compounds | Water, 105–180 °C, 5 MPa, 2.5 mL/min | Quercetin (15 mg/g onion skin) and quercetin-4-glucoside (8 mg/g onion skin) | [25] |
| Peach palm (4 g) |
Phenolic compounds, sugars | Water, 130 °C, 100 bar, 1 mL/min, 90 min | Soluble sugar (15 g/100 g), TPC (921 mg/100 g) | [26] |
| Shellfish waste (1 g) | Protein hydrolysates | Water, 200 °C, heating rate of 6 °C/min | 8.5 g protein/100 g dw (improved extraction yield of up to 65%) | [27] |
| Vine-canes (40 g) |
Phenolic compounds | Water, 250 °C, 50 min | 181 mg GAE/g dw, 203 mg TE/g dw | [28] |
| Vine co-products: cane, wood, and root (5 g) | Stilbenes | Water, 160 °C, 100 bar, 5 min | Cane (3.62 g/kg dw), wood (9.32 g/kg dw), and root (12.1 g/kg dw) | [29] |
| Pulsed electric fields | ||||
| Apple pomace (28.7 g) | Phenolic compounds | E = 2, 3 kV/cm, U = 17, 100 kJ/kg, 40 °C |
PEF performed with EtOH:H2O (70:30, v/v) showed the highest content of phlorizin (753.84 ± 26.38 µg/g fresh apple pomace) | [30] |
| Banana peels (-) |
Phenolic compounds | E = 1.3–6.45 kV/cm | Increase the TPC and antioxidant activity | [31] |
| Jackfruit waste (1:20 w/v solid-to-solvent ratio) | Pectin polysaccharide | E = 5–15 kV/cm | No significant effect on pectin yield | [32] |
| Lemon peels (30 g) | Phenolic compounds | E = 7 kV/cm, U = 7.6 kJ/kg | Increase the efficiency of phenolic compounds (hesperidin and eriocitrin) extraction by 300% | [33] |
| Potato peels (5 g) |
Phenolic compounds | E = 0.25–3 kV/ cm, U = 1–20 kJ/kg | PEF showed higher TPC yield (10%) and antioxidant activity (9%) compared to conventional solid–liquid extraction with same extraction protocol but without the application of the PEF pre-treatment) | [34] |
| Pomegranate peels (30 g) | Ellagic acid | E = 10 kV/cm | PEF selectively extracted and enhanced the recovery of ellagic acid (≈740 μg/g dm) | [35] |
| Pomelo peels (1 g) | Naringenin | E = 2–10 kV/cm | Increase the extraction yield of naringenin | [36] |
| Olive pomace (850 g) | Phenolic compounds | E = 1–6.5 kV/cm, U = 0.9–51.1 kJ/kg, 50 pulses spaced at 3 s, 20–27.5 °C | PEF allowed a 28.8% increased recovery yield of polyphenols (~3 mg GAE/L) compared to untreated | [37] |
| Tomato peels (10 g) | Lycopene | E = 5 kV/cm, U = 5 kJ/kg, 20 ± 2 °C | Enhance the extraction rate (27–37%), the lycopene yields (12–18%) and the antioxidant power (18–18.2%) | [38] |
| Ultrasound-assisted extraction | ||||
| Apple leaves (10 g) | Phloretin | 400 W, 20 kHz, 14.4 min, <25 °C | The phloretin concentration ranged from 292 to 726 µg/g | [39] |
| Apple pomace (1:10 (w/v) S:L ratio) | Phenolic compounds | 45 min, 45 °C | Increase the TPC, antioxidant activity, and recovery of interesting antioxidant compounds (quercetin derivatives, chlorogenic acid, phloridzin) | [40] |
| Beet leaves (1:20 (w/v) S:L ratio) | Bioactive compounds | 90 W, 16 min | Yields were 14.9 mg/g polyphenols, 949.1 µg/g betaxanthins, and 562.2 µg/g beta-cyanins | [41] |
| Brewers’ spent grains (1:30 (w/v) S:L ratio) | Proanthocyanidins | 400 w, 75 % acetone, 55 min, 25 °C | High recovery of proanthocyanidins (1023 µg/g dw) | [42] |
| Citrus peel (1 g) |
Citric acid | 119–141 W, 5.8–35.5 min, 0–7 % (v/v) ethanol | Recovery of 6.4 g and 3.4 g of citric acid per 100 g of dry orange and lime peels, respectively | [43] |
| Grape pomace (280 g) | Phenolic compounds | 450 W, 15 min, 20 °C | Increased the TPC (6.68 ± 0.05 mg of gallic acid) and antioxidant activity (ABTS: 23.84 ± 0.57 μmol of Trolox equivalents/g and DPPH: 33.27 ± 2.00 μmol of Trolox equivalents/g) | [44] |
| Kiwi peel (1.5 g) |
Flavonoids | 5–500 W, 20 kHz, 1–45 min, 25 °C | 46% extract weight and 1.51 mg/g dw of flavonoids | [45] |
| Orange peels (10 g) | Bioactive compounds | 40 kHz,85 min, 55 °C, 61% methanol | The spectra of extracts showed a similar fingerprint of hesperidin | [46] |
| Tomato peels (72 mL/g, L:S ratio) | Lycopene | 20 kHz, 20 min, 65 °C, | Lycopene recovery of 1536 µg/g | [47] |
| Microwave-assisted extraction | ||||
| Carrot juice waste (flaxseed oil + waste ~ 20 g) | Carotenoids | 170 W, 9.46 min, 8:1 g/g oil-to-waste ratio | Carotenoid recovery of 77.48%. The enriched flaxseed oil showed high phenolic content (214.05 ± 1.61 μg GAE/g oil) and antioxidant activity (inhibition % of DPPH = 70.67 ± 0.85) | [48] |
| Coffee pulp (-) |
Phenolic compounds, flavonoids, chlorogenic acid, and caffeine | 1000 W, 85 min, 1:100 g/100 mL sample-to-solvent ratio, 42.5 % (v/v) aqueous ethanol solution | Extraction yields of TPC, flavonoids, chlorogenic acid, and caffeine were 38.68, 27.00, 6.95, and 5.47 (mg/g dw), respectively. The extract showed high antioxidant capacities (ABTS, DPPH, and FRAP assays as 87.95, 9.3, 65.31 (mg TE/g DW), respectively) | [49] |
| Peach waste (1000 mg) |
Phenolic compounds and anthocyanins | 500 W, 90 s, 80 % ethanol (v/v) | TPC of 19.35 mg GAE/g fresh plant matter and total anthocyanin 1.12 mg cyn-3-glu/g fresh plant matter) yields | [50] |
| Cocoa shell waste (100 g) | β-Sitosterol | 500 W, 10 min, 70 °C | The maximum yield obtained was 13% higher than the yield of conventional maceration (3546.1 mg/ 100 g) | [51] |
| Eggplant peel (-) |
Phenolic compounds, flavonoids, anthocyanins | 269.82 W, 7.98 min, 5.01 mL/g L:S ratio | The maximum extraction yield (3.27%), TPC (1,049.84 µg GAE/mL), TFC (130.40 µg QE/mL), and total anthocyanin content (6.99 mg/L) | [52] |
| Lemon peel waste (-) |
Essential oil (limonene, β-pinene, and γ-terpinene) and pigment | 500 W, 50 min, 80 °C, 80% (v/v) ethanol, 1:10 L:S ratio | The extraction yields of lemon essential oil and pigment were around 2 wt.% and 6 wt.%, respectively | [53] |
| Spent sweet potato leaves (0.1 g) | Flavonoids | 470 W, 21 min, 54 °C, 70 mg/mL S:L ratio | The yield of TFC was 40.21 ± 0.23 mg rutin equivalents/g | [54] |
| Broccoli stems, leaves and florets (2.5 g) | Phenolic compounds (vanillic, sinapic, caffeic, chlorogenic, ferulic, gallic, neochlorogenic, and p-coumaric acids) | Stems: 2.45 GHz, 74.54% methanol, 15.9 min, 74.45 °C Leaves: 2.45 GHz, 80% methanol, 10 min, 73.27 °C Florets: 2.45 GHz, 80% methanol, 18.9 min, 75 °C |
MAE increased the phenolic yield up to 45.70% (1940.35 ± 0.794 µg GAE/g dw), for broccoli leaves, 133.57% (657.062 ± 0.771 µg GAE/g dw) for broccoli florets, and 65.30% for broccoli stems (225.273 ± 0.897 µg GAE/g dw), in less time compared with maceration extraction | [55] |
| Spent onion skins (-) | Flavonoids (quercetin, kaempferol, luteolin, and quercetin-3-O-β-D-glucoside) | 554 W, 16 min, 76 °C, 14 mg/mL S:L ratio | TFC extraction yields of 47.83 ± 0.21 mg/g | [56] |
| Enzyme-assisted extraction | ||||
| Unsold tomato (-) | Carotenoids and carotenoid-containing chromoplasts | Enzymatic mix: polygalacturonase, pectin lyase, cellulose, xylanase, 25 U/g for 180 min, 45−55 °C at pH 5–5.5 | Recovery yield of 4.30 ± 0.08 mg lycopene/ kg tomato)/U as carotenoid-containing chromoplasts and 5.43 ± 0.04 mg lycopene/ kg tomato)/U as total carotenoids | [57] |
| Apricot pulp (-) | Polysaccharides (sodium glycocholate and sodium taurocholate) | 5 mL/mg liquid-material ratio, 3% enzyme dosage and incubation time 1.5 h, pH 4.5 | The yield, sodium glycocholate and sodium taurocholate binding rates were 21.90%, 39.08% and 43.80%, respectively | [58] |
| Tomato peel and seed (4 g) | Lycopene-rich oleoresins | Enzymatic reaction: 40 °C, 5 h, 0.2 mL/g enzyme:substrate ratio, 5 mL/g solvent:substrate ratio, extraction time 1 h, 1 enzyme:enzyme ratio | Celluclast:Pectinex-ethyl acetate combination yielded the highest content of phenolic compounds (oleoresin with a concentration of 11.5 mg) | [59] |
| Beetroot cell wall (200 g) | Betalains | Enzymatic mix (cellulase 37%, xylanase 35%, pectinase 28%), 25 U/g total dose of enzymatic mix, 25 °C, 240 min, pH 5.5 | Betaxanthins and betacyanins yield 10 and 15 mg/mL U, respectively | [60] |
| Sweet cherry pomace (15 g) | Non-extractable polyphenols | 0.38 g/mL S:L ratio, 70 °C, pH 10, 40 min for Depol (90 µL/g of sample) and Promod (140 µL/g of sample) enzymes and 18.4 min for Pectinase enzyme (2 µL/g of sample) | The extracts obtained by acid hydrolysis (1.87 ± 0.05 mg GAE/g of extraction residue) and Promod enzyme (1.75 ± 0.20 mg GAE/g of extraction residue) followed by alkaline hydrolysis (1.46 ± 0.20 mg GAE/g of extraction residue) and enzymatic hydrolysis with Depol enzyme (1.33 ± 0.13 mg GAE/g of extraction residue) were the richest in terms of phenolic content | [61] |
| Sugar beet leaves | Protein | 54.25 °C, 81.35 min, 27.65 mL/g solvent/solid ratio | EAE increased the protein yield by 43.27% and reached a 79.01% yield | [62] |
| Raspberry pomace (9 g) | Lipophilic compounds (phytosterols) and polyphenols | 1.2 units of thermostable alkaline protease/100 g pomace press-cake, 60 °C, 2 h hydrolysis, pH 9 | The recovery of polyphenols and antioxidant activity was, respectively, 48% and 25% higher than the obtained by extraction with methanol/acetone/water mixture | [63] |
| Pressurized liquid extraction | ||||
| Pomegranate peel and carpelar (6 g) | Phenolic compounds (α, β punicalagin, and ellagic acid) | 60 °C, 80 bar, flow rate of 1 mL/min, 76 min, 10 solvent-to-feed ratio | The highest content of α, β-punicalagins, and ellagic acid obtained was 194.96 mg/100 g and 24.91 mg/100 g, respectively, representing 45% of TPC | [64] |
| Beetroot leaves and stems (5 g) | Phenolic compounds (ferulic acid, vitexin and sinapaldehyde) | 40 °C, 7.5, 10 and 12.5 MPa, flow rate of 3 mL/min | The highest TPC was obtained for beetroot leaves and varied from 7 ± 1 to 252 ± 2 mg GAE/g extract | [65] |
| Vitis vinifera L. cv. negra criolla pomace (5 g) | Phenolic compounds (flavanols and phenolic acids) | 10 atm, 5 min with 250 s of nitrogen purge Flavanols: 20% ethanol, 160 °C Phenolic acids: 60% ethanol, 160 °C |
PLE recovered ~2.5 and ~1.5 more polyphenols from skins (6.93 µg/g dw) and seeds (45.34 µg/g dw), respectively, compared to conventional extraction | [66] |
| Olive pomace (5 g) | Phenolic compounds (phenolic alcohols, secoiridoids, flavonoids, and lignans) | Clean-step with n-hexane as the solvent and 1500 psi at room temperature to remove the lipophilic fraction from the olive pomace. Ethanol (0 to 100%), 40 to 176 °C, 1500 psi, 20 min | PLE showed higher TPC than conventional extraction (1659 mg/kg dw and 281.7 mg/kg dw, respectively) | [67] |
| Pomegranate peel (3.75 g) | Phenolic compounds (phenolic acids, flavonoids, and hydrolysable tannins) | 200 °C, ethanol 77%, 1500 psi, 20 min | TPC of 164.3 ± 10.7 mg GAE/g dw | [68] |
| Pomegranate seed (1.75 g of waste and 7 g of sand, 1:4 ratio) | Protein and phenolic compounds | Ethanol (0 to 100 %), 28 to 170 °C, 1 to 5 cycles, 3 to 12 min, pH 6.5 to 11, 103 bar | Higher extraction yield by PLE (15.3 ± 0.9 g proteins/100 g pomegranate seed waste) at a cost of a longer extraction time and the co-extraction of phenolic compounds | [69] |
2.2. Subcritical Water Extraction
2.3. Pulsed Electric Fields
2.4. Ultrasound-Assisted Extraction
2.5. Microwave-Assisted Extraction
This entry is adapted from the peer-reviewed paper 10.3390/pr11010020
