Cancer is one of the major deadly diseases globally. The alarming rise in the mortality rate due to this disease attracks attention towards discovering potent anticancer agents to overcome its mortality rate. Based on their particular activity, a number of other plant-derived bioactive compounds are in the clinical development phase against cancer, such as gimatecan, elomotecan, etc. Additionally, the conjugation of natural compounds with anti-cancerous drugs, or some polymeric carriers particularly targeted to epitopes on the site of interest to tumors, can generate effective targeted treatment therapies.
- natural products
- anticancer drugs
- medicinal plants
1. Introduction
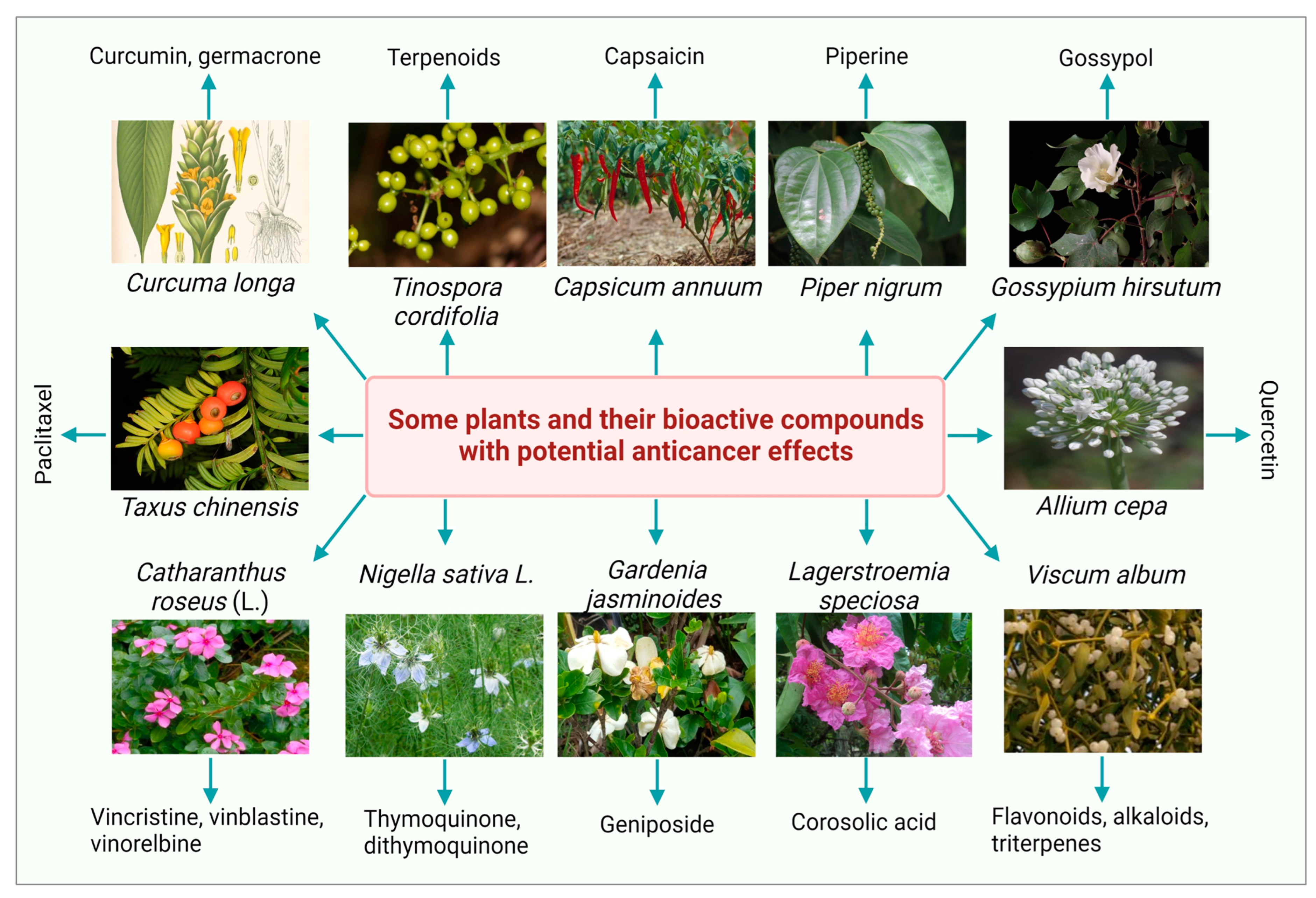
2. Plant-Derived Bioactive Compounds as Anti-Cancerous Agents
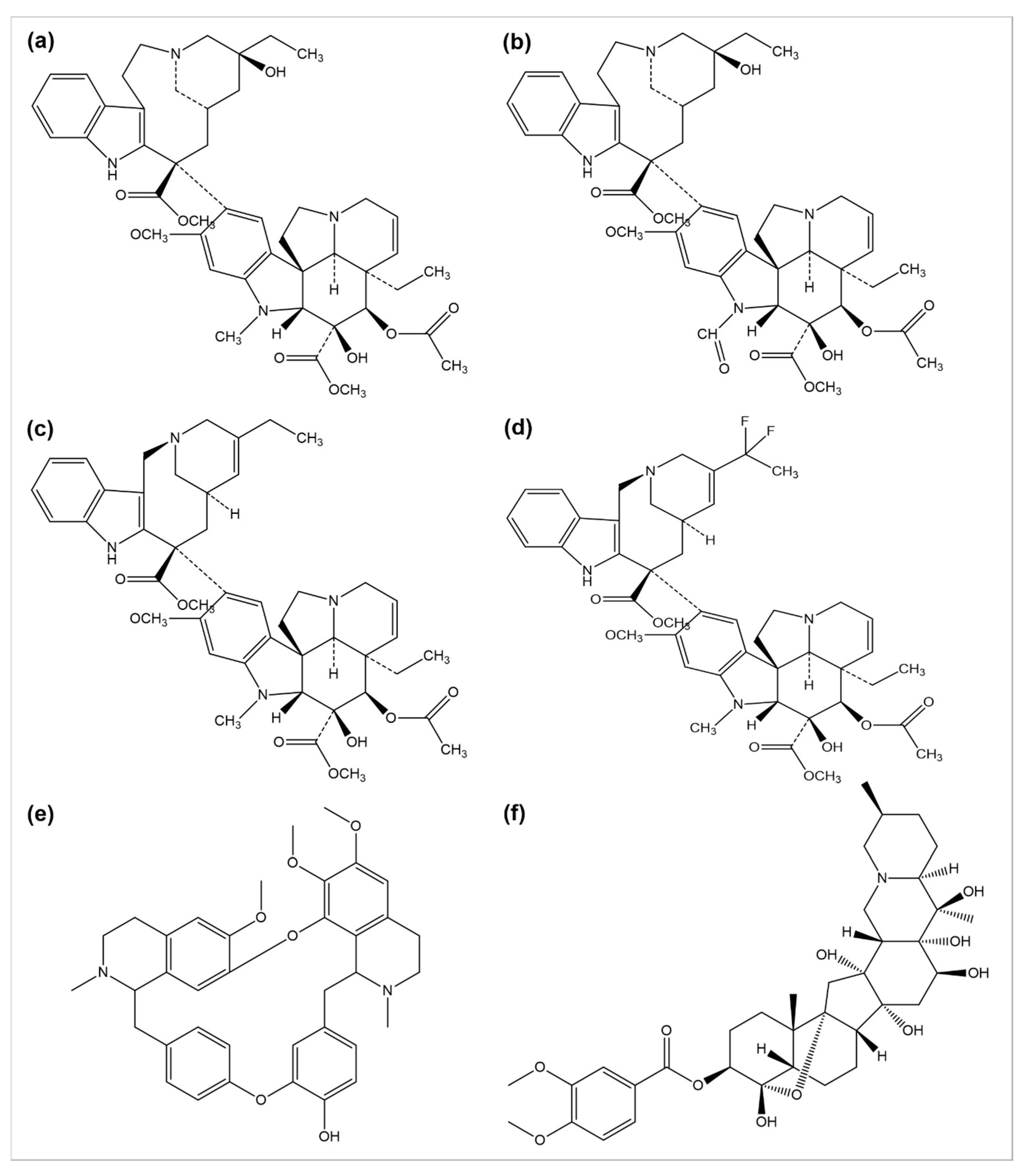
2.1. Vinca Alkaloids and Their Derivatives
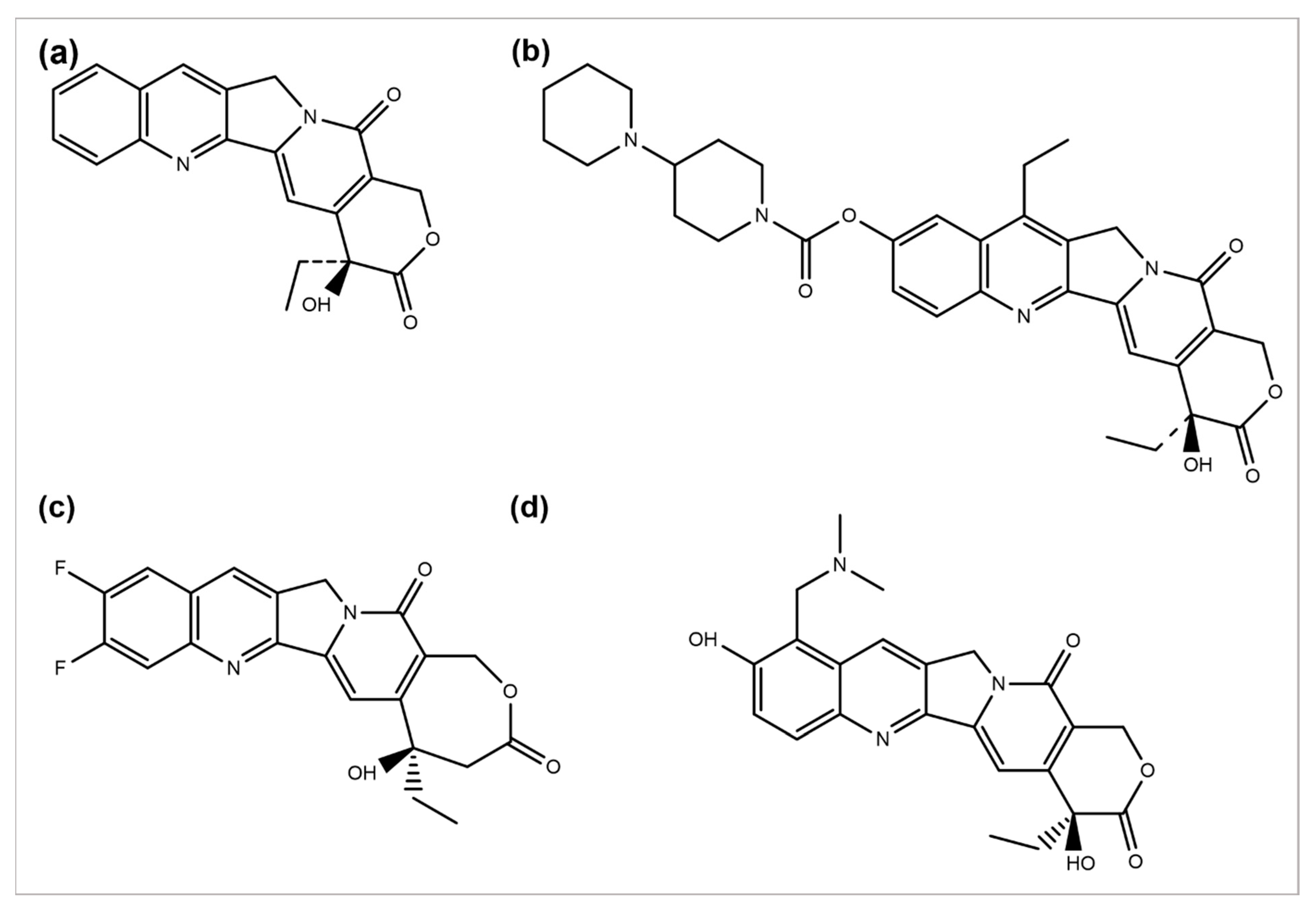
2.2. Camptothecin and Its Derivatives
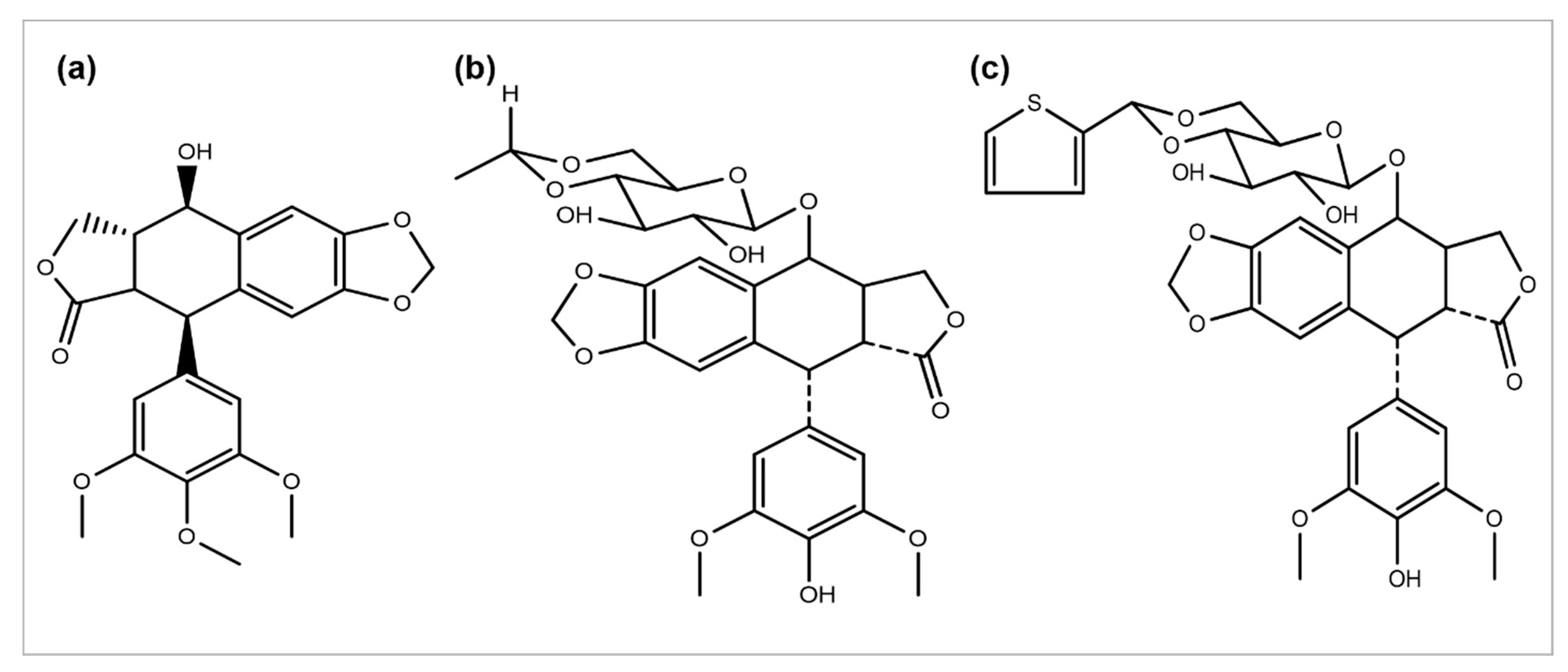
2.3. Podophyllotoxin and Its Semi-Synthetic Analogs
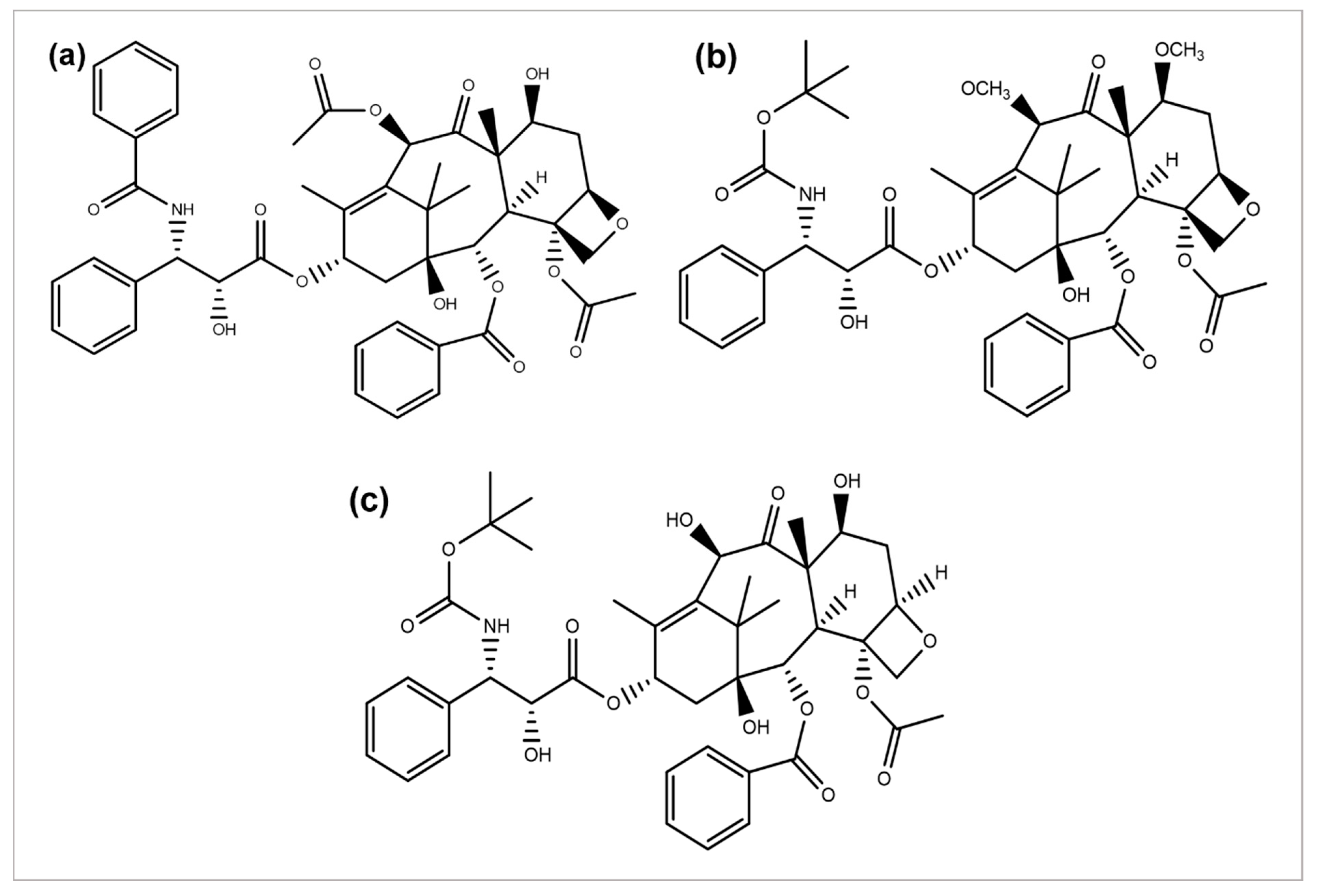
2.4. Taxane Diterpenoids
This entry is adapted from the peer-reviewed paper 10.3390/cancers14246203
References
- Tan, G.; Gyllenhaal, C.; Soejarto, D. Biodiversity as a source of anticancer drugs. Curr. Drug Targets 2006, 7, 265–277.
- De Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009, 123, 439–445.
- Hong, W.K.; Sporn, M.B. Recent advances in chemoprevention of cancer. Science 1997, 278, 1073–1077.
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59.
- Srivastava, V.; Negi, A.S.; Kumar, J.; Gupta, M.; Khanuja, S.P. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–5908.
- Newman, D.J.; Cragg, G.M. The discovery of anticancer drugs from natural sources. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2005; pp. 129–168.
- Larkin, T. Herbs are often more toxic than magical. FDA Consumers 1983, 17, 4–10.
- Saxe, T. Toxicity of medicinal herbal preparations. Am. Fam. Physician 1987, 35, 135–142.
- Madhuri, S.; Pandey, G. Some dietary agricultural plants with anticancer properties. Plant Arch. 2008, 8, 13–16.
- Sivalokanathan, S.; Ilayaraja, M.; Balasubramanian, M. Efficacy of Terminalia arjuna (Roxb.) on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Indian J. Exp. Biol. 2005, 43, 264–267.
- Chaudhary, A.; Singh, N. Contribution of world health organization in the global acceptance of Ayurveda. J. Ayurveda Integr. Med. 2011, 2, 176–186.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258.
- Semeniuc, C.A.; Socaciu, M.-I.; Socaci, S.A.; Mureșan, V.; Fogarasi, M.; Rotar, A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules 2018, 23, 2261.
- Pengelly, A.; Bone, K. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine; Routledge: Abingdon, UK, 2020.
- Ahmad, I.; Mehmood, Z.; Mohammad, F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998, 62, 183–193.
- Singla, R.K.; De, R.; Efferth, T.; Mezzetti, B.; Sahab Uddin, M.d.; Sanusi; Ntie-Kang, F.; Wang, D.; Schultz, F.; Kharat, K.R.; et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through #INPST Hashtag Analysis. Phytomedicine 2023, 108, 154520.
- Rosangkima, G.; Prasad, S. Antitumour activity of some plants from Meghalaya and Mizoram against murine ascites Dalton's lymphoma. Indian J. Exp. Biol. 2004, 42, 981–988.
- Cragg, G.M.; Newman, D.J. Plants as a source of anticancer agents. J. Ethnopharmacol. 2005, 100, 72–79.
- Sohn, H.; Okos, M.R. Paclitaxel (taxol): From nutt to drug. J. Microbiol. Biotechnol. 1998, 8, 427–440.
- Kaur, R.; Kapoor, K.; Kaur, H. Plants as a source of anticancer agents. J. Nat. Prod. Plant Resour. 2011, 1, 119–124.
- Fouché, G.; Cragg, G.; Pillay, P.; Kolesnikova, N.; Maharaj, V.; Senabe, J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008, 119, 455–461.
- Georgaki, S.; Skopeliti, M.; Tsiatas, M.; Nicolaou, K.A.; Ioannou, K.; Husband, A.; Bamias, A.; Dimopoulos, M.A.; Constantinou, A.I.; Tsitsilonis, O.E. Phenoxodiol, an anticancer isoflavene, induces immunomodulatory effects in vitro and in vivo. J. Cell Mol. Med. 2009, 13, 3929–3938.
- Kinghorn, A.; Farnsworth, N.; Beecher, C.; Soejarto, D.; Cordell, G.; Pezzuto, J.; Wall, M.; Wani, M.; Brown, D.; O'neill, M.M.; et al. Novel strategies for plant-derived anticancer agents. Int. J. Pharmacogn. 1995, 33, 48–58.
- Kinghorn, A.D.; Su, B.-N.; Jang, D.S.; Chang, L.C.; Lee, D.; Gu, J.-Q.; Carcache-Blanco, E.J.; Pawlus, A.D.; Lee, S.K.; Park, E.J. Natural inhibitors of carcinogenesis. Planta Med. 2004, 70, 691–705.
- Powis, G. Anticancer Drugs: Antimetabolite Metabolism and Natural Anticancer Agents, 1st ed.; Pergamon: Oxford, UK, 1994; Section 140.
- Yun, T.K. Update from Asia: Asian studies on cancer chemoprevention. Ann. N. Y. Acad. Sci. 1999, 889, 157–192.
- Kamath, K.; Park, K. Mucosal adhesive preparations. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1988; Volume 10, p. 133.
- Sagini, K.; Urbanelli, L.; Buratta, S.; Leonardi, L.; Emiliani, C. Nanovesicles from plants as edible carriers of bioactive compounds. AgroLife Sci. J. 2017, 6, 167–171.
- Shengquan, L.; Ngong, H.S. Design of low-molecular-weight prodrugs for targeted delivery of anticancer agents. In Proceedings of the 3rd International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems, Chicago, IL, USA, 8–10 April 2013.
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug strategies in anticancer chemotherapy. ChemMedChem 2008, 3, 20–53.
- Sporn, M.B.; Liby, K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005, 2, 518–525.
- Ertas Onmaz, N.; Demirezen Yilmaz, D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green synthesis of gold nanoflowers using Rosmarinus officinalis and Helichrysum italicum extracts: Comparative studies of their antimicrobial and antibiofilm activities. Antibiotics 2022, 11, 1466.
- Verma, A.; Singh, R. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian J. Pharm. Sci. 2010, 72, 655–657.
- Jordan, M.A.; Thrower, D.; Wilson, L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991, 51, 2212–2222.
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265.
- Mamtani, R.; Vaughn, D.J. Vinflunine in the treatment of advanced bladder cancer. Expert Rev. Anticancer Ther. 2011, 11, 13–20.
- Bachner, M.; De Santis, M. Vinflunine in the treatment of bladder cancer. Ther. Clin. Risk Manag. 2008, 4, 1243–1253.
- Bennouna, J.; Delord, J.-P.; Campone, M.; Nguyen, L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008, 14, 1625–1632.
- Kruczynski, A.; Barret, J.-M.; Etiévant, C.; Colpaert, F.; Fahy, J.; Hill, B.T. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem. Pharmacol. 1998, 55, 635–648.
- Pourroy, B.; Carré, M.; Honoré, S.; Bourgarel-Rey, V.; Kruczynski, A.; Briand, C.; Braguer, D. Low concentrations of vinflunine induce apoptosis in human SK-N-SH neuroblastoma cells through a postmitotic G1 arrest and a mitochondrial pathway. Mol. Pharmacol. 2004, 66, 580–591.
- Simoens, C.; Vermorken, J.B.; Korst, A.E.; Pauwels, B.; De Pooter, C.M.; Pattyn, G.G.; Lambrechts, H.A.; Breillout, F.; Lardon, F. Cell cycle effects of vinflunine, the most recent promising Vinca alkaloid, and its interaction with radiation, in vitro. Cancer Chemother. Pharmacol. 2006, 58, 210–218.
- Cao, Z.; Zhu, S.; Xue, Z.; Zhang, F.; Zhang, L.; Zhang, Y.; Guo, Y.; Zhan, G.; Zhang, X.; Guo, Z. Isoquinoline alkaloids from Hylomecon japonica and their potential anti-breast cancer activities. Phytochemistry 2022, 202, 113321.
- Freeling, J.L.; Scholl, J.L.; Eikanger, M.; Knoblich, C.; Potts, R.A.; Anderson, D.J.; Rower, J.E.; Farjoo, M.H.; Zhao, H.; Pillatzki, A. Pre-clinical safety and therapeutic efficacy of a plant-based alkaloid in a human colon cancer xenograft model. Cell Death Discov. 2022, 8, 135.
- Liu, L.; Yan, J.; Cao, Y.; Yan, Y.; Shen, X.; Yu, B.; Tao, L.; Wang, S. Proliferation, migration and invasion of triple negative breast cancer cells are suppressed by berbamine via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol. Lett. 2021, 21, 70.
- Esnaashari, S.S.; Muhammadnejad, S.; Amanpour, S.; Amani, A. A combinational approach towards treatment of breast cancer: An analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech 2020, 21, 166.
- Hsiang, Y.-H.; Hertzberg, R.; Hecht, S.; Liu, L. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878.
- Oberlies, N.H.; Kroll, D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004, 67, 129–135.
- Pazdur, R.; Diaz-Canton, E.; Ballard, W.P.; Bradof, J.E.; Graham, S.; Arbuck, S.G.; Abbruzzese, J.L.; Winn, R. Phase II trial of 9-aminocamptothecin administered as a 72-hour continuous infusion in metastatic colorectal carcinoma. J. Clin. Oncol. 1997, 15, 2905–2909.
- Farray, D.; Ahluwalia, M.S.; Snyder, J.; Barnett, G.H.; Cohen, B.H.; Suh, J.H.; Peereboom, D.M. Pre-irradiation 9-amino camptothecin (9-AC) in patients with newly diagnosed glioblastoma multiforme. Invest. New Drugs. 2006, 24, 177–180.
- Scott, L.; Soepenberg, O.; Verweij, J.; de Jonge, M.; Th Planting, A.; McGovern, D.; Principe, P.; Obach, R.; Twelves, C. A multicentre phase I and pharmacokinetic study of BN80915 (diflomotecan) administered daily as a 20-min intravenous infusion for 5 days every 3 weeks to patients with advanced solid tumours. Ann. Oncol. 2007, 18, 569–575.
- Zhu, A.X.; Ready, N.; Clark, J.W.; Safran, H.; Amato, A.; Salem, N.; Pace, S.; He, X.; Zvereva, N.; Lynch, T.J. Phase I and pharmacokinetic study of gimatecan given orally once a week for 3 of 4 weeks in patients with advanced solid tumors. Clin. Cancer Res. 2009, 15, 374–381.
- Pecorelli, S.; Ray-Coquard, I.; Tredan, O.; Colombo, N.; Parma, G.; Tisi, G.; Katsaros, D.; Lhomme, C.; Lissoni, A.; Vermorken, J. Phase II of oral gimatecan in patients with recurrent epithelial ovarian, fallopian tube or peritoneal cancer, previously treated with platinum and taxanes. Ann. Oncol. 2009, 21, 759–765.
- Trocóniz, I.F.; Cendrós, J.-M.; Soto, E.; Pruñonosa, J.; Perez-Mayoral, A.; Peraire, C.; Principe, P.; Delavault, P.; Cvitkovic, F.; Lesimple, T. Population pharmacokinetic/pharmacodynamic modeling of drug-induced adverse effects of a novel homocamptothecin analog, elomotecan (BN80927), in a Phase I dose finding study in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 239–250.
- Kurzrock, R.; Goel, S.; Wheler, J.; Hong, D.; Fu, S.; Rezai, K.; Morgan-Linnell, S.K.; Urien, S.; Mani, S.; Chaudhary, I. Safety, pharmacokinetics, and activity of EZN-2208, a novel conjugate of polyethylene glycol and SN38, in patients with advanced malignancies. Cancer 2012, 118, 6144–6151.
- Imbert, T. Discovery of podophyllotoxins. Biochimie 1998, 80, 207–222.
- Sargent, J.M.; Elgie, A.W.; Williamson, C.J.; Hill, B.T. Ex vivo effects of the dual topoisomerase inhibitor tafluposide (F 11782) on cells isolated from fresh tumor samples taken from patients with cancer. Anti Cancer Drugs 2003, 14, 467–473.
- Kinghorn, A.D.; Seo, E.-K. Plants as sources of drugs. In Agricultural Materials as Renewable Resources; Fuller, G., McKeon, T.A., Bills, D.D., Eds.; ACS Publications: Washington, DC, USA, 1996; Volume 647, pp. 179–193.
- Gelmon, K. The taxoids: Paclitaxel and docetaxel. The Lancet 1994, 344, 1267–1272.
- Bissery, M.-C.; Nohynek, G.; Sanderink, G.-J.; Lavelle, F. Docetaxel (Taxotere): A review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs 1995, 6, 339–355, 363–368.
- Beaulieu, E.; Demeule, M.; Ghitescu, L.; Béliveau, R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997, 326, 539–544.
- Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhänel, M.; Spruß, T.; Bernhardt, G.; Graeff, C.; Färber, L.; Gschaidmeier, H. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Invest. 2002, 110, 1309–1318.
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin. Cancer Res. 2003, 9, 2849–2855.
- Paller, C.J.; Antonarakis, E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011, 5, 117–124.
- Bao, R.; Chan, P. Novel compounds in the treatment of lung cancer: Current and developing therapeutic agents. J. Exp. Pharmacol. 2011, 3, 21–34.
