Viruses innately have the ability to mutate constantly and lead to variants. Some variants emerge and disappear while some persist. Mutations to the virus happen during the process of viral replication, that is when the virus attaches to the ACE2 receptor, which is present on the membrane of the host cell.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), better known as Coronavirus (COVID-19), is an infectious disease that is said to have firstly originated in Wuhan, China [
1]. The World Health Organization (WHO) first learned of this new virus on 31 December 2019, following the reporting of a cluster of cases of ‘viral pneumonia’ in Wuhan, People’s Republic of China [
2]. By the end of January 2020, the WHO officially declared COVID-19 as a pandemic, a Public Health Emergency of International Concern (PHEIC). The virus spread to around 25 countries by early February 2020, the spreading of the virus was due to the lack of action taken in many parts of the world. The spread of the virus since then was rapid and currently, COVID-19 cases are present worldwide in 213 countries, areas, or territories. Hence, guidelines and criteria for diagnosis, treatment, and preventative measures had to be established rapidly due to the increasing number of people that were getting infected with the virus [
3]. Researchers worldwide worked and shared their contributions regarding the epidemiology, prevention, treatment, clinical and diagnostic patterns of the COVID-19 virus. Viral detection using RT-PCR identified the SARS-CoV-2 virus to be the disease that has caused this viral transmission worldwide [
3].
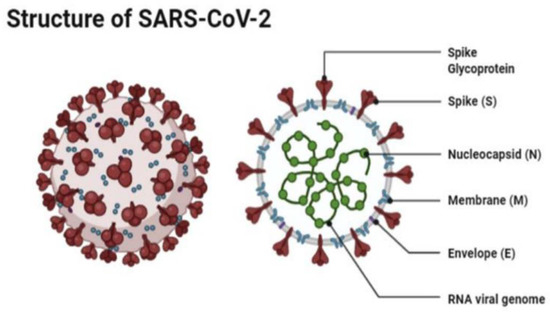
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a beta coronavirus that belongs to the Coronaviridae family. The family is composed of single-stranded positive ribonucleic acid (RNA) viruses [
4]. The size of the virus is between 50 and 150 nm in diameter, and its linearity and positive-sense RNA genome are large. SARS-CoV-2 is an enveloped spherical-shaped virus. It has four structural proteins and 16 nonstructural proteins. The structural proteins are the nucleocapsid (N) protein, the membrane (M), the S protein, and the envelope (E) protein. The ribonucleic acid (RNA) is oriented in a 5′-3′direction which makes it a positive sense RNA virus, and the RNA can be read directly as a messenger RNA [
4]. The RNA replicase is encoded at the 5′ terminal end. The nonstructural protein 14 (nsp14) has proofreading activity which allows the rate of mutations to stay low. The S protein causes the attachment of the virus to the host cell at the angiotensin-converting enzyme 2 (ACE2) receptor, which is present on the membrane of the host cell. The ACE2 receptors are found in abundance on alveolar cells [
4] (
Figure 1).
Figure 1. The structure of SAR-CoV-2 under a microscope as illustrated by Agarwal et al. [
5].
Most infected people will develop mild to moderate respiratory illness and recover without requiring special treatment or hospitalization. Nevertheless, some infected Patients with COVID-19 can develop pneumonia, severe symptoms of acute respiratory distress syndrome (ARDS), and multiple organ failure [
6]. Patients with underlying medical conditions such as cardiovascular disease, diabetes, chronic respiratory disease, and cancer, and older people are more likely to develop serious illness. However, Epidemiological studies have shown that mortalities are higher in the elder population and the incidence is much lower in children [
7]. COVID-19 affects different people in different ways. Symptoms of COVID-19 consist of two states, the (i) symptomatic state, and the (ii) asymptomatic state. The symptomatic state can be noticed through the patient showing multiple different symptoms such as fever and/or cough [
8]. The less common symptoms of COVID-19 include sore throat, headache, aches, pains, diarrhoea, a rash on the skin, discoloration of fingers or toes, and red or irritated eyes. While the more serious symptoms of COVID-19 are difficulty breathing (due to the lowering of oxygen levels) or shortness of breath, loss of speech or mobility, or confusion and chest pain. The transmission of the virus occurs if a person touches a surface contaminated with SARS-CoV-2, and then the hands come into direct contact with mucous membranes such as the eyes, nose, or mouth [
9]. Although it is more common that transmission occurs through symptomatic patients, asymptomatic patients who show no symptoms of the virus due to the immune system’s capability of combatting the virus, are the main source of transmission; through their respiratory droplets being airborne, as well as transmitted through virus contaminated containers and foods [
10]. Therefore, rapid contact tracing and testing that identifies asymptomatic cases are conducted [
11]. Similar to other coronaviruses, SARS-CoV-2 can be established by multiple virus genotypes. This genetic diversity can lead to some advantages for the virus, such as better binding to the receptor, faster replication, and more effective suppression or avoidance of the host’s immune response [
12].
Vaccines:
With the economic, societal and public health effects of COVID-19, it was essential to develop vaccine to minimize the severe consequences of this virus. Before the development of the vaccines, some non- pharmaceutical interventions have shown benefits in minimizing the spread of COVID-19. Those non-pharmaceutical interventions included social distancing, wearing of the facemasks and limits of large gatherings. However, they had limited effectiveness due to poor adherence with those practices and unclear advices from ministries of public health. Therefore, the development of an effective COVID-19 vaccine has been a critical need to control the disease and its effects (18).
Global collaboration among pharmaceutical companies, governments and academic researchers were mounted to develop COVID-19 vaccine since a publication about the SARS-CoV-2 viral sequence was released on January 10, 2020. The three main authorities that coordinated the vaccine research were World Health Organization, Gavi and coalition for Epidemic Preparedness and Innovation (CEPI). It was reported by CEPI that there were 321 vaccine candidates in development around the globe in September 2020. However, only 40 vaccine candidates have progressed to clinical trials in humans in October 2020 and 11 of them were in phase 3 clinical trials that aims to provide the safety and efficacy evidence that is required for approval of the vaccine.
This literature review aims to analyze all the vaccine candidates that went to phase II and above with the rationale behind the completion of some vaccines to phase III and why others failed to continue to phase III. Moreover, this paper will provide comprehensive information about COVID-19 infection, its symptoms, the structure of the virus, the variants that we have become aware off and how they differ. Furthermore, it will inform the literature about vaccines that developed for COVID-19 infection, vaccine vehicles and trials on vaccines. This paper shall open doors for further research and better understanding for reasons that hinder vaccines from reaching the market, which is important to be taken into consideration when developing new vaccines for viruses in the future.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10122086