Water scarcity is an increasing problem on every continent, which instigated the search for novel ways to provide clean water suitable for human use; one such way is desalination. Desalination refers to the process of purifying salts and contaminants to produce water suitable for domestic and industrial applications. Due to the high costs and energy consumption associated with some desalination techniques, membrane-based technologies have emerged as a promising alternative water treatment, due to their high energy efficiency, operational simplicity, and lower cost. However, membrane fouling is a major challenge to membrane-based separation as it has detrimental effects on the membrane’s performance and integrity. Based on the type of accumulated foulants, fouling can be classified into particulate, organic, inorganic, and biofouling. Biofouling is considered the most problematic among the four fouling categories.
1. Introduction
Clean water is a finite resource with continuously growing demand; according to UN-Water, around 2.3 billion people live in water-stressed countries, of which 733 million live in high and critically water-stressed countries [
1]. Desalination provides a way to produce fresh water more suitable for human use and agriculture from saline or brackish water [
2]. Due to reductions in production costs and time, membrane-based separation in water treatment has gained increased popularity over the past few decades. Commonly used membrane separation technologies include reverse osmosis (RO), micro- and ultrafiltration, membrane distillation (MD), and electrodialysis (ED), while membrane crystallization (MCr) and pervaporation (PV) are still under laboratory evaluation [
2,
3,
4].
Membrane technology has been extensively evaluated for water desalination. With respect to economic analyses of the costs and energy consumption associated with these methods, Nthunya et al. [
3] provided a comprehensive review in which they compared the capital and operating expenditures (CAPEX and OPEX) of RO, ED, MD, and MCr for different-sized desalination processes of brackish water and seawater. In their analysis, capital costs encompassed design costs, transport, equipment, project management, instrumentation, infrastructure, and buildings. In comparison, operation, and maintenance (O&M) costs included labor, insurance, energy, maintenance, and consumables. Their findings showed that O&M costs were lower for ED compared to the other membrane techniques investigated, and the small-scale MD OPEX was lower than that for RO. However, there is not much reported on the large-scale O&M of MD due to its slow industrial growth. Accordingly, they concluded that RO is the most preferrable membrane separation process in the current water desalination markets. Kesieme et al. [
2] conducted an economic analysis comparing MD and RO. Considering a reference 30,000 m
3/day plant, RO was economically favorable even with the inclusion of the carbon tax ($23 per ton carbon) in Australia. However, if heat is available at low costs, the cost of MD would have been reduced to $0.66/m
3, which is cheaper than RO. The authors raised an important point regarding carbon emissions taxes, stating that with policies coming into practice to tax carbon emissions, the economics of these membrane processes will undergo changes that introduce uncertainties in costing reports as to what desalinated water will cost in a carbon-constrained society.
In membrane processes, the membrane is always in contact with the solutions being treated. Consequently, it is prone to chemical or biological deposition of matter. Membrane fouling refers to the deposition and accumulation of materials on or in the membrane. It results from complex interactions between the various foulants in the feed and the membrane surface [
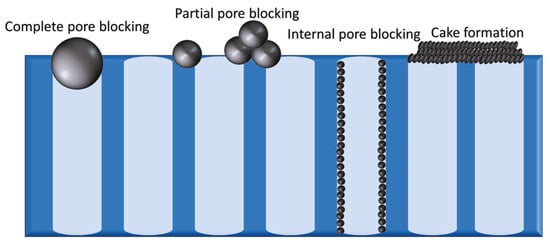
5]. There are four types of fouling, namely, complete pore blocking, partial pore blocking, internal pore blocking, and cake formation (
Figure 1). In order to control these types, the nature of the foulants present must be known; accordingly, membrane foulants can be categorized into particulate, organic, inorganic, and biofoulants [
6,
7,
8,
9,
10,
11].
Figure 1. The four types of fouling (from left to right) complete pore blocking, partial pore blocking, internal pore blocking, and cake formation.
Biofouling, which involves the accumulation of biological microorganisms followed by the formation of a biofilm on the membrane [
6,
12], can be divided into microfouling and macrofouling. Microfouling involves the accumulation of unicellular or multicellular microorganisms (e.g., bacteria, yeast, or fungi) that may form a biofilm by mono-species or multi-species, whereas macrofouling is associated with bigger organisms, such as algae [
10]. Biofouling accounts for approximately 45% of membrane fouling and is generally regarded as the most problematic among the four fouling categories [
5].
The topic of membrane fouling has been investigated intensely in literature, with numerous reviews having been written on the topic. Some of these reviews are focused on the general aspects of membrane fouling. For example, Guo and Ngo [
9] identified the major foulants, the principal membrane fouling mechanisms, as well as possible mitigation processes. Charcosset [
11] reviewed the main characteristics of membrane processes, membrane fouling, energy consumption, and associated environmental issues. In comparison, Rudolph et al. [
13] presented a review of the state-of-the-art techniques used for in situ membrane monitoring. In other reviews, membrane fouling was examined in relation to a particular membrane separation method; for instance, Shi et al. [
14] reviewed the different techniques available for predicting fouling in membrane bioreactors. Qasim et al. [
4] provided an extensive review of the theories and models underlying membrane transport, and membrane fouling. They also provided a thorough discussion of the different membrane cleaning and pretreatment technologies, in addition to current challenges faced by RO membrane processes. Hubadillah et al. [
15] reported on alternative techniques to RO, such as forward osmosis (FO) and MD. With respect to artificial intelligence (AI), Bagheri et al. [
16], Lim et al. [
17], in addition to Viet and Jang [
18], presented a general overview of the application of AI to membrane fouling prediction.
2. Membrane Biofouling Mechanism
Membrane biofouling occurs in three main steps: attachment, propagation, and biofilm formation. The first step involves the deposition and physical adsorption of microorganisms (algae, protozoa, bacteria, and fungi) to the membrane surface. This process is controlled by three main factors [
22,
23,
24,
25]:
-
Microorganism: this includes species, population density, growth profile, nutrient status, the hydrophobicity/hydrophilicity of the microorganism, and physiological responses.
-
Surface morphology: membrane material, surface charge, hydrophobicity, roughness, and porosity.
-
Feed: temperature, pH, dissolved organic/inorganic matter, shear forces, and flux.
Hydrodynamic forces or physicochemical interactions can drive the adhesion of microorganisms to the surface. The convective nature of hydrodynamic forces brings suspended microorganisms and other foulants close to the membrane surface, facilitating their adhesion to the surface. Physicochemical interactions can be divided into long-range Lifshitz–van der Waals interactions, and short-range Lewis acid–base and electrostatic double-layer interactions [
6,
21]. Adsorption is followed by cell growth and multiplication [
19,
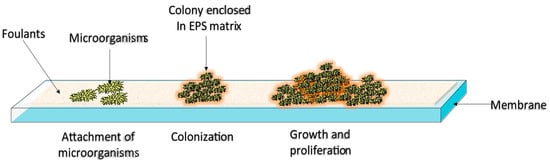
22]. During the colonization and proliferation stage, the attached microorganisms secrete a polymeric material known as the extracellular polymeric substance (EPS) matrix, which further anchors them to the membrane [
23] (refer to
Figure 2). The EPS matrix composition is highly dependent upon the environment in which the biofilm develops; however, EPS matrices usually contain organic molecules (e.g., proteins, nucleic acids, lipids, and polysaccharides), and inorganic matter (e.g., minerals, clay, and corrosion products). Among the EPS matrix components, proteins play a significant role in biofouling because they provide an optimum environment for microbial colonization, i.e., the building blocks of proteins (amino acids) possess several functional groups, such as carboxyl, amino, and methyl groups. The presence of these functional groups affects the hydrophilicity of proteins, which in turn influences the adhesion of the EPS-enclosed microorganisms to the membrane through diverse intramolecular forces, such as van der Waals forces, hydrogen bonding, and hydrophobic interactions [
6,
8,
10,
26,
27].
Figure 2. Biofouling mechanism.
The impact of biofouling on the membrane processes includes flux decline, damage to the membrane structure, inhibition of conventional transport mechanisms, increased feed and differential pressure, increased energy consumption, and the need for frequent cleaning, which adversely affects membrane plant operation and shortens membrane life [
22,
28].
3. Membrane Biofouling Characterization
The characterization of membrane biofouling involves evaluating the microbial community, the fouling layer, and the quantification of the EPS matrix. Various assays, microscopic and spectroscopic techniques have been employed to characterize membrane biofouling [
29]. Kerdi et al. [
30] used 3D-optical coherence tomography (OCT) to characterize the intrinsic structure and the mechanical properties of the biofilm developing on ultrafiltration (UF) polyethersulfone membranes without altering their chemical and/or physical properties. Three-dimensional images of the biofilm were obtained with high resolution, enabling the biofilm microstructural morphology analysis. The structural properties were found to be dependent of time as the biofilm continuously evolved, i.e., the biofilm was more elastic in nature at the initial stages of its growth. Still, it then transitioned into a more viscoelastic type as it matured. Benladghem et al. [
31] used surface enhanced Raman spectroscopy (SERS) to identify biofoulants accumulated on spiral-wound reverse osmosis (SWRO) membranes. They also imaged the fouled membranes’ topography and the biofilm structures using fluorescence microscopy (FM) and scanning electron microscopy (SEM). The microscopy images showed that both biotic and abiotic deposits were present on the membrane; in addition, SERS showed that the thickness of the fouling layer reached up to 5µm. In another study, Zahid et al. [
32] used thermal analysis (differential scanning calorimetry, DSC), contact angle, SEM, Fourier transform infrared (FTIR) spectroscopy, and the disc diffusion method to characterize cellulose acetate (CA)-sulfonated graphene oxide (SGO)-doped membranes and assess their anti-biofouling properties. The functionalization of CA membranes with SGO was confirmed using FTIR, while the morphology of the doped membranes was studied using SEM. The thermal stability of the doped membranes was investigated using DSC and revealed an increase in thermal stability of CA-SGO membranes upon the addition of SGO.
Escherichia coli pathogenic bacteria were used in the disc/agar well diffusion test through measurement of inhibition zone. The test results showed that that antibacterial activity of the CA membrane is increased with increased SGO content of SGO. This behavior was attributed to the fact that the surface of CA-SGO-doped membranes had a negative charge from the sulfonic acid and hydroxyl groups of SGO, which led to electrostatic repulsion between the microorganisms and the membrane. Masigol et al. [
33] developed an interesting technique to characterize and identify multi-species biofilms termed polymer surface dissection (PSD). PSD uses targeted and ultraviolet (UV)-responsive polyethylene glycol hydrogels to bind to and detach microorganism aggregates from membranes. The detached aggregates are then exposed to UV light to release aggregates of the desired size for DNA extraction. The efficacy of PSD was demonstrated by identifying the bacterial community structure (aggregate area of 5000−60,000 μm
2) developed during early-stage biofouling of aerobic wastewater communities over polyvinylidene difluoride (PVDF) membranes. The findings showed that larger aggregates had less bacterial diversity. Moreover, the bacterial community structure shifted from one rich in Bacteroides to one with more proteobacteria when the aggregates areas reached the 25,000–45,000 μm
2 size range. The efficiency of bacterial transfer between the membrane surface and the hydrogel still poses an issue for the developed method. The authors proposed using poly-Llysine (PLL) as a targeting ligand to improve transfer efficiency, as well as the application of external electric fields to enhance transfer efficiency. It is important to note that for EPS matrix characterization, the EPS matrix needs to be extracted first, which can be done using physical methods (e.g., centrifugation, dialysis, filtration, ion exchange, heating), chemical methods (commonly used chemicals include, sodium hydroxide, formaldehyde, ethanol) or a combination of both [
5,
23,
34].
This entry is adapted from the peer-reviewed paper 10.3390/membranes12121271