Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The anaphase-promoting complex/cyclosome (APC/C) is a complicated cellular component that plays significant roles in regulating the cell cycle process of eukaryotic organisms. The spatiotemporal regulation mechanisms of APC/C in distinct cell cycle transitions are no longer mysterious, and the components of this protein complex are gradually identified and characterized.
- APC/C
- cell cycle
- aging
- lifespan
1. Introduction
The ability of cells to replicate themselves accurately is crucial to the life and development of all organisms. One of the most important regulatory factors, the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase that specifically targets cell cycle-related proteins for degradation, exhibits essential functions in the regulation of the eukaryotic cell cycle, particularly during anaphase entry and mitotic exit [1][2][3]. The subunits of APC/C are largely conserved from yeast to humans, principally organized into three subcomplexes: the catalytic core (APC2, APC10 and APC11), the tetratricopeptide repeat lobe (APC3, APC6, APC7 and APC8) and the platform (APC1, APC4, APC5 and APC15) subcomplex [4][5]. Two key APC/C activators, Cdc20 and Cdh1, which determine most of its substrate selectivity, control APC/C activity in a cell cycle-dependent manner [6]. In addition, inhibitors, the mitotic checkpoint complex (MCC) and phosphatases interact with the APC/C to spatially and temporally modulate its activity and ensure the accurate execution of mitotic events [7][8][9][10]. During the G1 phase, APC/CCdh1 is an active complex. With the accumulation of G1-cyclins, Cdh1 becomes phosphorylated and separates from the APC/C. This phosphorylation and APC/CCdh1 inactivity will be continued to anaphase [11]. From G2 to prophase, free APC/C is inactivated by its inhibitor Emi1, which associates with Cdc20 and prevents APC/C-Cdc20 binding [7]. At late prophase, Emi1 is proteolyzed, and RASSFA1 takes over the role of this inhibitor until late prometaphase, when the latter is also degraded [12]. Free APC/C is then phosphorylated by Polo-like kinase 1 (Plk1) and cyclin B/cdk1 [13]. At metaphase, APC/CCdc20 is still inactivated owing to the direct binding of the MCC. Once the spindle checkpoint is satisfied, the MCC is separated from APC/CCdc20, and this protein complex achieves its full activity and then induces the proteolysis of securin and cyclin B [3][8]. Degradation of securin actives separase and disassociates sister chromatids from each other by cleaving cohesin complexes, achieving metaphase to anaphase transition [14]. At the same time, continuous cyclin B degradation at anaphase induces dephosphorylation of Cdh1 and a decreased cyclin B activity, which is important for mitotic exit. Meanwhile, Cdc20 is degraded in an APC/CCdh1-dependent manner [3][11]. Thus, the APC/C is a key factor in the regulation of the cell cycle. With a deepening understanding of APC/C, however, it has been widely recognized that APC/C functions include more than mitosis.
Ageing, which is broadly defined as the progressive decline in homeostasis and functional integrity, has attracted great attention and has been a subject of curiosity throughout human history. People have reached some consensus on its hallmarks, such as cellular senescence, genome instability and loss of proteostasis [15][16]. Cellular senescence means the onset of body ageing for multicellular organisms but the end of their reproduction and even death for unicellular organisms. In yeast, various APC/C mutants cause different degrees of cell cycle arrest [17][18][19][20], which is a characteristic of senescent cells [21][22]. APC/C prevents chromosomal aneuploidy by precisely regulating cell cycle progression [23][24], thus, maintaining genomic stability. As a member of the ubiquitin-proteasome system (UPS), the APC/C also plays an important role in the proteostasis network [25]. Additionally, the insufficient function of the APC/C has been observed in ageing-related disease models, including Alzheimer’s disease (AD), premature ageing and cancer models [26][27][28]. Thus, the APC/C seems to have a great relevance with ageing.
2. The Normal Operation of the APC/C Is Crucial to the Lifespan
On the basis of comprehensive knowledge of this unicellular organism and advanced tools for research into its physiology, budding yeast has become an ideal model organism to study ageing mechanisms in recent years. Sirtuins, mTOR signalling and dietary restrictions are considered key conserved longevity components from yeast to vertebrates [29][30][31]. Moreover, many cellular ageing regulators remain to be discovered.
The APC/C shows great relevance to genome instability and cancer [23], revealing a potential role for APC/C in ageing. In fact, deficient APC/C subunits (apc5CA, apc9∆, apc10∆ or cdc26∆) led to various degrees of shortened replicative lifespan (RLS) in yeast [32]. The apc10∆ mutant caused the most serious lifespan defect, probably due to its crucial role in the catalytic core and substrate recognition of the APC/C [33]. Interestingly, the lifespan of the apc5CA apc10∆ double mutant was shorter than either the apc5CA or apc10∆ mutant alone, and overexpression of APC5 reduced yeast lifespan [32]. Apc5p is a strict stoichiometric component of the APC/C since reduced or elevated levels of Apc5p were found to reduce the yeast lifespan.
In addition to yeast, APC/C deficiency exacerbates ageing in other species. For instance, the absence of CDC26 destroyed the human oocyte maturation process and led to oocyte ageing, while these defects were partially rescued by overexpression of Cdc26p [34]. Mice lacking Cdh1 entered replicative ageing prematurely due to the stabilization of Ets2 and subsequent activation of p16Ink4a expression and caused early lethality, revealing an essential role for APC/C in maintaining the RLS of murine embryonic fibroblasts [35]. Interestingly, abnormal activation of the APC/C in mammalian cells also induced ageing, similar to its effect on yeast reported in a previous study [32]. Kuo et al. found that premature activation of the APC/C by T-lymphotropic virus type 1 Tax induced rapid senescence independent of pRb or p53 activity [36]. Mitosis skipping mediated by the p53-dependent premature activation of APC/CCdh1 was necessary and sufficient for senescence induction [37]. Moreover, loss of Emi1-dependent APC/C inhibition elicited DNA damage-induced senescence [38]. Cdh1 is essential for the functions of APC/C in neuronal survival [39] and is tightly regulated by its own degradation, which depends upon two RXXL-type destruction boxes [40]. In human cells, the APC/C inhibitor MAD2L2 sequesters Cdh1 to prevent premature APC/C activation prior to anaphase onset, thereby contributing to mitotic fidelity [41]. Taken together, these studies showed that the normal function of the APC/C is of great significance in cellular senescence from yeasts to humans.
Cell differentiation is an essential process for the growth, development, reproduction, and longevity of all multicellular organisms [42]. The APC/C is also involved in regulating this process by mediating cell cycle withdrawal and promoting certain differentiation-related license factors synthesis [43]. By degradation of Skp2 to stabilize p27, which in turn downregulates Cdks activities, APC/CCdh1 elongates the G1 phase or G0 arrest to coordinate cell type-specific differentiation processes [44]. In response to TGF-β (transforming growth factor beta) stimulation, Smad3 can recruit APC/CCdh1 to ubiquitinate SnoN (Ski-related novel protein N), leading to its degradation and activation of TGF-β target genes and growth inhibition [45]. APC/CCdh1 also targets Id (inhibitor of differentiation/DNA binding) proteins, leading to activation of bHLH (basic helix-loop-helix) transcription factors and its target gene expression, which mediate differentiation in various cell types [46]. However, how the cell cycle and cell cycle-independent functions of APC/C are regulated during development remains poorly understood.
3. Mechanisms of the APC/C in Regulating Lifespan
To researchers' knowledge, the APC/C regulates lifespan through at least the following two mechanisms: maintaining genomic stability and regulating the stress response (Figure 1).
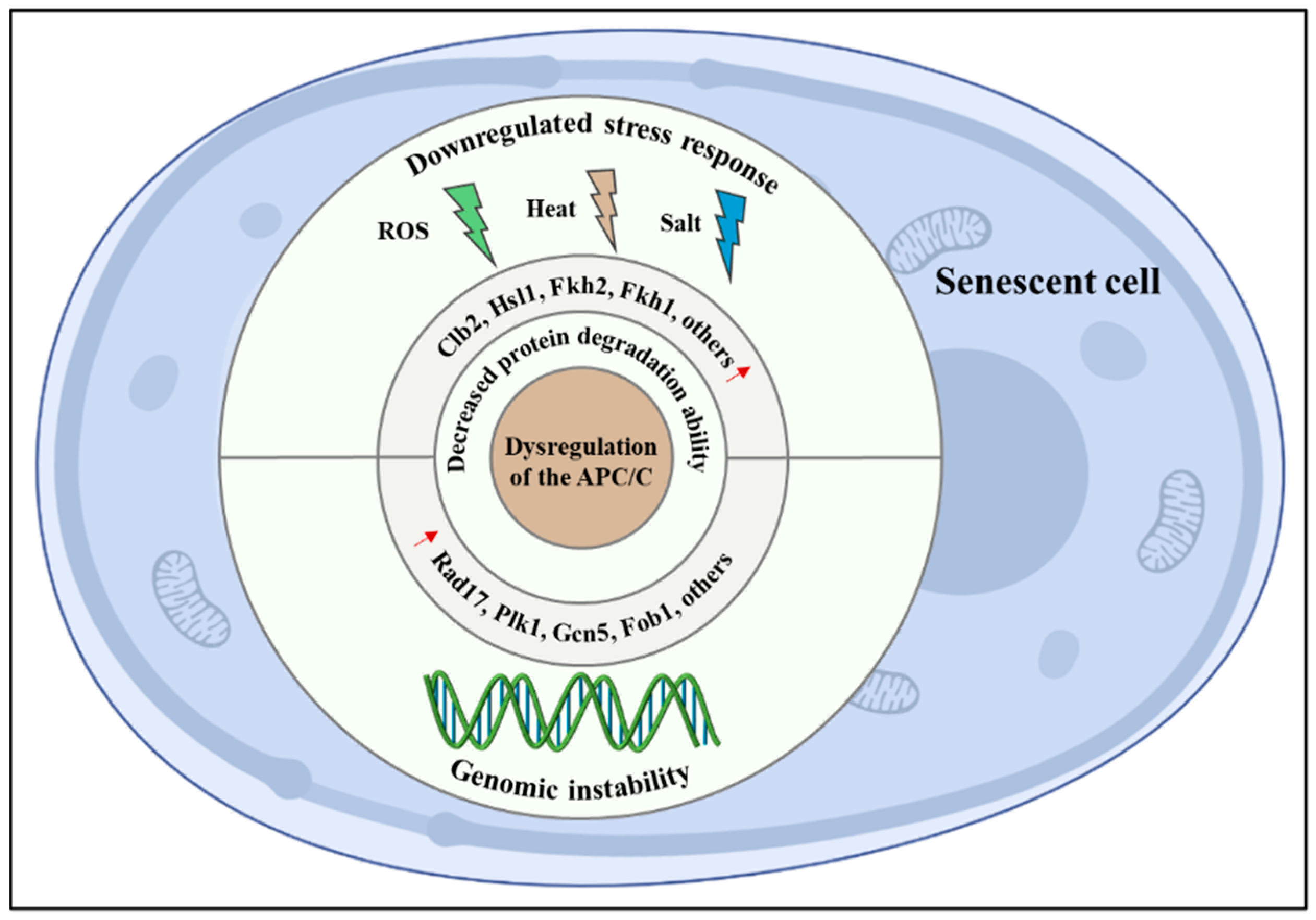
Figure 1. Mechanisms of the anaphase-promoting complex/cyclosome (APC/C) in regulating lifespan. Dysregulation of APC/C can trigger the abnormal accumulation of its substrates, thus, leading to genomic instability and downregulated stress response. Some lifespan determinants, such as Fob1 and Fkh1, are both identified as a bona fide APC/C substrate, indicating that APC/C promotes longevity at least partly owing to the degradation of them.
3.1. Maintaining Genomic Stability
Genomic instability is a commonly accepted feature of ageing [15]. During normal ageing, genome stability and integrity are continuously challenged by numerous endogenous genotoxins, including DNA replication errors, reactive oxygen species and spontaneous hydrolytic reactions, as well as exogenous threats, such as chemical, physical, and biological agents [47]. Organisms have evolved a complex network of DNA repair mechanisms to deal with this damage collectively [48], but these repair mechanisms appear to be defective during cellular ageing [49]. Some premature ageing syndromes, such as Bloom syndrome and Werner syndrome, have been reported to be related to DNA damage accumulation [50]. In addition, genomic instability is associated with nuclear lamina deficits and aged mitochondrial DNA mutations [51].
Studies have shown that Cdh1 deficiency in mammalian cells caused genomic instability, such as structural and numerical chromosomal aberrations in mouse embryonic fibroblasts and chromosome separation and cytokinesis aberrations in primary human cells [52][53]. However, the exact causes of these phenotypes are unclear. Recently, several protein regulators of genomic stability and DNA damage repairs, such as Rad17, ubiquitin-specific protease 1, and Claspin, as well as the proteins G9a and Glucagon-like peptide, have been shown to be bona fide APC/CCdh1 substrates [54][55][56][57]. While Cdh1 overexpression improves emotion and cognitive-related behaviours in global cerebral ischemia rats, indicating that Cdh1 abundance exerts a neuroprotective effect [58]. Endoreduplication is a process of nuclear genome replication in the absence of mitosis, which leads to elevated nuclear gene content and polyploidy [59]. APC/CCdh1 is re-activated after the S phase resulting in reduced Cdk activity, thereby mediating the transition of mitotic cycles to endoreduplication cycles [60]. Altering SlCCS52A (ortholog of Cdh1 in plants) expression in either a positive or negative manner impacts the extent of endoreduplication in fruit and affects fruit size [61]. MAD2L2 plays an important role in several processes, such as DNA double-strand break repair, translesion synthesis and mitosis [62][63]. It is not only the APC/C regulator but also the substrate, which is rapidly degraded by APC/CCdc20 at the onset of anaphase, releasing Cdh1 to activate the dephosphorylated APC/C [41]. Therefore, it is likely that more unidentified APC/C substrates play roles in DNA damage repair and genome integrity, further underlining the importance and relevance of APC/C in maintaining cellular genomic stability.
In addition, activation of APC/CCdh1 was significant to the DNA damage-induced G2 checkpoint in chicken cells [64] and later observed in human cells [56]. Although APC/CCdh1 was active only during the G1 phase and mitotic exit, it was reactivated in the G2 phase in response to genotoxic stress to target the mitotic kinase Plk1, which shows clear potential for facilitating aberrant chromosome separation and DNA replication to delay degradation and prevent mitotic entry until damaged DNA has been repaired [56]. In APC/CCdh1-deficient cells, mitotic entry can still be delayed because of other existing G2-phase DNA damage checkpoints [56], and the APC/CCdh1-dependent checkpoint is not functional in these cells. Thus, cells with impaired DNA may enter mitosis more easily but with more risks, eventually leading to genetic lesions.
Recent studies have suggested that the APC/C plays a role in chromatin assembly and histone modifications [17][65], which are both required for DNA damage repair [66][67]. Based on the genetic interactions between the APC/C with Asf1 and CAF-1 mutants [68], the combinations lead to worsened phenotypes and can be reversed by elevating the expression of Asf1 or any CAF-1 subunits; the APC/C may be involved in DNA damage repair in a chromatin assembly dependent manner. In budding yeast, multiple APC/C subunit mutants showed reduced levels of H3K56Ac, H3K9Ac and H3K79Me [65]. H3K56Ac is important for DNA repair and histone deposition [69], H3K9Ac is also required for transcriptional activation [70], and H3K79Me is involved in various activities, such as the regulation of transcription, the cell-cycle checkpoint, DNA repair and cellular development [71]. Thus, the reduced levels of these modifications owing to an impaired APC/C could have a great influence on DNA repair, chromatin and chromosome structure, and transcription. Moreover, the histone acetyltransferase (HAT) GCN5, which is involved in centromere maintenance, DNA repair and transcriptional elongation [72], interacts with the APC/C genetically and functionally and has been shown to be targeted by the APC/C for degradation during the M/G1 transition [65]. Except in yeast, the APC/C has been reported to be involved in the mitotic turnover of TRRAP (TRansformation domain-Associated Protein), a common component of the HAT complex [73].
The molecular mechanisms that determine yeast lifespan have been extensively studied, and one of the important factors has been found to be Fob1 [74][75]. Fob1 antagonistically interacts with Sir2, leading to the accumulation of extrachromosomal ribosomal DNA circles (ERCs) [75], which were the first asymmetrically inherited form of molecular damage identified to cause ageing in yeast. Studies have demonstrated that APC/C plays a role in rDNA silencing, assembly and segregation [76][77]. Recently, Fob1 has been identified as a bona fide APC/C substrate [78]. Deletion of FOB1 suppressed APC/C-mutant phenotypes, including decreased RLS, increased the rDNA recombination rate and the number of cell cycle defects [78], suggesting that the APC/C maintains genomic stability and, thus, promotes longevity, partially owing, at least, to the degradation of Fob1.
3.2. Regulating the Stress Response
Increasing evidence demonstrates that stress, such as genotoxic and oxidative stress, shows a strong relationship with ageing [79][80]. Organisms evolve a series of repair mechanisms to prevent long-term damage, but the balance between the stress response and repair pathways is disrupted during ageing, causing an increased rate of ageing and age-related pathologies [81]. In addition, previous studies have indicated that many of the pathways that modulate stress resistance (such as the PKA, mTOR and Sch9 pathways) also play essential roles in lifespan regulation [31][82].
The Forkhead box (FOX) transcription factor family exhibits a conserved function in the regulation of stress responses and the cell cycle [83]. The APC/C genetically interacts with Fkh proteins, as indicated by the deletion of FKH1 and FKH2, exacerbating the effects of APC/C-mutant phenotypes, such as reduced lifespan and increased oxidative and temperature stress sensitivity, which can be reversed by increasing FKH expression [84][85]. Owing to their genetic redundancy, only deletion of both FKH1 and FKH2 reduces yeast lifespan in a Forkhead box O (FOXO)-like manner [84]. The Fkh1 protein is degraded specifically during mitosis in a proteasome- and APC/CCdc20-dependent manner, while a stable Fkh1 mutant exhibits increased stress sensitivity and genomic instability and has been associated with a decreased normal lifespan [85]. In fact, targeting of Fox proteins by the APC/C is a conserved process, as indicated by the mammalian Forkhead box M1 (FOXM1) also being identified as a target of the APC/CCdh1 during late mitosis and the early G1 phase for degradation, which is important for regulated entry into the S phase [86].
Several studies have shown that APC/C-defect mutants are sensitive to multiple stresses. In budding yeast, the cdh1∆ mutant was sensitive to caffeine, ethanol and salt [87], and cells lacking CDC26 were sensitive to elevated temperature [18]. The accumulation of Hsl1 and Clb2, two APC/C substrates that can disrupt MAPK pathway signalling, induced stress sensitivity, indicating that the APC/C may enhance stress resistance by inhibiting inhibitory signalling [87]. Moreover, the APC/C participates in an acute response to protein-damaging stress by mediating ubiquitination and degradation of heat shock factor 2 (HSF2) and supports cell survival in response to endoplasmic reticulum stress by Cdh1-dependent degradation of its substrates [88][89]. In Caenorhabditis elegans, multiple APC/C loss-of-function mutants showed supersensitive phenotype to aldicarb [90], whose responsiveness can be indirectly reflected the muscle activity [91], indicating APC/C can inhibit muscle excitation at the neuromuscular junction.
The list of APC/C substrates continues to increase, as does the number of discoveries showing its involvement in cellular functions. Therefore, the continued study of APC/C substrates involved in regulating lifespan will provide new insight into the role of these substrates in cellular ageing.
This entry is adapted from the peer-reviewed paper 10.3390/ijms232315327
References
- Morgan, D.O. Regulation of the APC and the exit from mitosis. Nat. Cell Biol. 1999, 1, E47–E53.
- Peters, J.M. The anaphase promoting complex/cyclosome: A machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006, 7, 644–656.
- Sivakumar, S.; Gorbsky, G.J. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat. Rev. Mol. Cell Biol. 2015, 16, 82–94.
- Chang, L.F.; Zhang, Z.; Yang, J.; McLaughlin, S.H.; Barford, D. Molecular architecture and mechanism of the anaphase-promoting complex. Nature 2014, 513, 388–393.
- Acquaviva, C.; Pines, J. The anaphase-promoting complex/cyclosome: APC/C. J. Cell Sci. 2006, 119, 2401–2404.
- Visintin, R.; Prinz, S.; Amon, A. CDC20 and CDH1: A family of substrate-specific activators of APC-dependent proteolysis. Science 1997, 278, 460–463.
- Reimann, J.D.; Freed, E.; Hsu, J.Y.; Kramer, E.R.; Peters, J.M.; Jackson, P.K. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 2001, 105, 645–655.
- Sudakin, V.; Chan, G.K.; Yen, T.J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001, 154, 925–936.
- Rudner, A.D.; Murray, A.W. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 2000, 149, 1377–1390.
- Peters, J.M. The anaphase-promoting complex: Proteolysis in mitosis and beyond. Mol. Cell 2002, 9, 931–943.
- Castro, A.; Bernis, C.; Vigneron, S.; Labbé, J.C.; Lorca, T. The anaphase-promoting complex: A key factor in the regulation of cell cycle. Oncogene 2005, 24, 314–325.
- Song, M.S.; Song, S.J.; Ayad, N.G.; Chang, J.S.; Lee, J.H.; Hong, H.K.; Lee, H.; Choi, N.; Kim, J.; Kim, H.; et al. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat. Cell Biol. 2004, 6, 129–137.
- Moshe, Y.; Boulaire, J.; Pagano, M.; Hershko, A. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. USA 2004, 101, 7937–7942.
- Nasmyth, K. Separating sister chromatids. Trends Biochem. Sci. 1999, 24, 98–104.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713.
- Harkness, T.A.; Davies, G.F.; Ramaswamy, V.; Arnason, T.G. The ubiquitin-dependent targeting pathway in Saccharomyces cerevisiae plays a critical role in multiple chromatin assembly regulatory steps. Genetics 2002, 162, 615–632.
- Hartwell, L.H.; Mortimer, R.K.; Culotti, J.; Culotti, M. Genetic control of the cell division cycle in yeast: V. genetic analysis of cdc mutants. Genetics 1973, 74, 267–286.
- Heichman, K.A.; Roberts, J.M. The yeast CDC16 and CDC27 genes restrict DNA replication to once per cell cycle. Cell 1996, 85, 39–48.
- Leverson, J.D.; Joazeiro, C.A.; Page, A.M.; Huang, H.; Hieter, P.; Hunter, T. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 2000, 11, 2315–2325.
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827.
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 2018, 28, 436–453.
- Naylor, R.M.; van Deursen, J.M. Aneuploidy in cancer and aging. Annu. Rev. Genet. 2016, 50, 45–66.
- Wang, H.; Feng, K.; Wang, Q.; Deng, H. Reciprocal interaction between SIRT6 and APC/C regulates genomic stability. Sci. Rep. 2021, 11, 14253.
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464.
- Fujita, H.; Sasaki, T.; Miyamoto, T.; Akutsu, S.N.; Sato, S.; Mori, T.; Nakabayashi, K.; Hata, K.; Suzuki, H.; Kosaki, K.; et al. Premature aging syndrome showing random chromosome number instabilities with CDC20 mutation. Aging Cell 2020, 19, e13251.
- Fuchsberger, T.; Lloret, A.; Vina, J. New functions of APC/C ubiquitin ligase in the nervous system and its role in Alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1057.
- Wang, Q.; Moyret-Lalle, C.; Couzon, F.; Surbiguet-Clippe, C.; Saurin, J.C.; Lorca, T.; Navarro, C.; Puisieux, A. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 2003, 22, 1486–1490.
- Wasko, B.M.; Kaeberlein, M. Yeast replicative aging: A paradigm for defining conserved longevity interventions. FEMS Yeast Res. 2014, 14, 148–159.
- Khan, A.H.; Zou, Z.; Xiang, Y.; Chen, S.; Tian, X.L. Conserved signaling pathways genetically associated with longevity across the species. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1745–1755.
- Kaeberlein, M. Lessons on longevity from budding yeast. Nature 2010, 464, 513–519.
- Harkness, T.A.; Shea, K.A.; Legrand, C.; Brahmania, M.; Davies, G.F. A functional analysis reveals dependence on the anaphase-promoting complex for prolonged life span in yeast. Genetics 2004, 168, 759–774.
- Passmore, L.A.; McCormack, E.A.; Au, S.W.; Paul, A.; Willison, K.R.; Harper, J.W.; Barford, D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003, 22, 786–796.
- Li, L.; Xia, Y.; Yang, Y.; Zhang, W.; Yan, H.; Yin, P.; Li, K.; Chen, Y.; Lu, L.; Tong, G. CDC26 is a key factor in human oocyte aging. Hum. Reprod. 2021, 36, 3095–3107.
- Li, M.; Shin, Y.H.; Hou, L.; Huang, X.; Wei, Z.; Klann, E.; Zhang, P. The adaptor protein of the anaphase promoting complex Cdh1 is essential in maintaining replicative lifespan and in learning and memory. Nat. Cell Biol. 2008, 10, 1083–1089.
- Kuo, Y.L.; Giam, C.Z. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 2006, 25, 1741–1752.
- Johmura, Y.; Shimada, M.; Misaki, T.; Naiki-Ito, A.; Miyoshi, H.; Motoyama, N.; Ohtani, N.; Hara, E.; Nakamura, M.; Morita, A.; et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol. Cell 2014, 55, 73–84.
- Verschuren, E.W.; Ban, K.H.; Masek, M.A.; Lehman, N.L.; Jackson, P.K. Loss of Emi1-dependent anaphase-promoting complex/cyclosome inhibition deregulates E2F target expression and elicits DNA damage-induced senescence. Mol. Cell Biol. 2007, 27, 7955–7965.
- Almeida, A. Regulation of APC/C-Cdh1 and its function in neuronal survival. Mol. Neurobiol. 2012, 46, 547–554.
- Listovsky, T.; Oren, Y.S.; Yudkovsky, Y.; Mahbubani, H.M.; Weiss, A.M.; Lebendiker, M.; Brandeis, M. Mammalian Cdh1/Fzr mediates its own degradation. EMBO J. 2004, 23, 1619–1626.
- Listovsky, T.; Sale, J.E. Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset. J. Cell Biol. 2013, 203, 87–100.
- Sánchez Alvarado, A.; Yamanaka, S. Rethinking differentiation: Stem cells, regeneration, and plasticity. Cell 2014, 157, 110–119.
- Qiao, X.; Zhang, L.; Gamper, A.M.; Fujita, T.; Wan, Y. APC/C-Cdh1: From cell cycle to cellular differentiation and genomic integrity. Cell Cycle 2010, 9, 3904–3912.
- Wei, W.; Ayad, N.G.; Wan, Y.; Zhang, G.J.; Kirschner, M.W.; Kaelin, W.G., Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 2004, 428, 194–198.
- Stroschein, S.L.; Bonni, S.; Wrana, J.L.; Luo, K. Smad3 recruits the anaphase-promoting complex for ubiquitination and degradation of SnoN. Genes Dev. 2001, 15, 2822–2836.
- Lasorella, A.; Stegmüller, J.; Guardavaccaro, D.; Liu, G.; Carro, M.S.; Rothschild, G.; de la Torre-Ubieta, L.; Pagano, M.; Bonni, A.; Iavarone, A. Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 2006, 442, 471–474.
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485.
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294.
- Young, T.Z.; Liu, P.; Urbonaite, G.; Acar, M. Quantitative Insights into Age-Associated DNA-Repair Inefficiency in Single Cells. Cell Rep. 2019, 28, 2220–2230.e7.
- Burtner, C.R.; Kennedy, B.K. Progeria syndromes and ageing: What is the connection? Nat. Rev. Mol. Cell Biol. 2010, 11, 567–578.
- Kauppila, T.E.S.; Bratic, A.; Jensen, M.B.; Baggio, F.; Partridge, L.; Jasper, H.; Gronke, S.; Larsson, N.G. Mutations of mitochondrial DNA are not major contributors to aging of fruit flies. Proc. Natl. Acad. Sci. USA 2018, 115, E9620–E9629.
- Garcia-Higuera, I.; Manchado, E.; Dubus, P.; Canamero, M.; Mendez, J.; Moreno, S.; Malumbres, M. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat. Cell Biol. 2008, 10, 802–811.
- Engelbert, D.; Schnerch, D.; Baumgarten, A.; Wasch, R. The ubiquitin ligase APCCdh1 is required to maintain genome integrity in primary human cells. Oncogene 2008, 27, 907–917.
- Zhang, L.; Park, C.H.; Wu, J.; Kim, H.; Liu, W.; Fujita, T.; Balasubramani, M.; Schreiber, E.M.; Wang, X.F.; Wan, Y. Proteolysis of Rad17 by Cdh1/APC regulates checkpoint termination and recovery from genotoxic stress. EMBO J. 2010, 29, 1726–1737.
- Cotto-Rios, X.M.; Jones, M.J.; Busino, L.; Pagano, M.; Huang, T.T. APC/CCdh1-dependent proteolysis of USP1 regulates the response to UV-mediated DNA damage. J. Cell Biol. 2011, 194, 177–186.
- Bassermann, F.; Frescas, D.; Guardavaccaro, D.; Busino, L.; Peschiaroli, A.; Pagano, M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 2008, 134, 256–267.
- Takahashi, A.; Imai, Y.; Yamakoshi, K.; Kuninaka, S.; Ohtani, N.; Yoshimoto, S.; Hori, S.; Tachibana, M.; Anderton, E.; Takeuchi, T.; et al. DNA damage signaling triggers degradation of histone methyltransferases through APC/CCdh1 in senescent cells. Mol. Cell 2012, 45, 123–131.
- Zhang, B.; Chen, X.; Lv, Y.; Wu, X.; Gui, L.; Zhang, Y.; Qiu, J.; Song, G.; Yao, W.; Wan, L.; et al. Cdh1 overexpression improves emotion and cognitive-related behaviors via regulating hippocampal neuroplasticity in global cerebral ischemia rats. Neurochem. Int. 2019, 124, 225–237.
- Larkins, B.A.; Dilkes, B.P.; Dante, R.A.; Coelho, C.M.; Woo, Y.M.; Liu, Y. Investigating the hows and whys of DNA endoreduplication. J. Exp. Bot. 2001, 52, 183–192.
- Eguren, M.; Manchado, E.; Malumbres, M. Non-mitotic functions of the anaphase-promoting complex. Semin. Cell Dev. Biol. 2011, 22, 572–578.
- Mathieu-Rivet, E.; Gévaudant, F.; Cheniclet, C.; Hernould, M.; Chevalier, C. The anaphase promoting complex activator CCS52A, a key factor for fruit growth and endoreduplication in tomato. Plant Signal. Behav. 2010, 5, 985–987.
- de Krijger, I.; Föhr, B.; Pérez, S.H.; Vincendeau, E.; Serrat, J.; Thouin, A.M.; Susvirkar, V.; Lescale, C.; Paniagua, I.; Hoekman, L.; et al. MAD2L2 dimerization and TRIP13 control shieldin activity in DNA repair. Nat. Commun. 2021, 12, 5421.
- Pernicone, N.; Grinshpon, S.; Listovsky, T. CDH1 binds MAD2L2 in a Rev1-like pattern. Biochem. Biophys. Res. Commun. 2020, 531, 566–572.
- Sudo, T.; Ota, Y.; Kotani, S.; Nakao, M.; Takami, Y.; Takeda, S.; Saya, H. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 2001, 20, 6499–6508.
- Turner, E.L.; Malo, M.E.; Pisclevich, M.G.; Dash, M.D.; Davies, G.F.; Arnason, T.G.; Harkness, T.A. The Saccharomyces cerevisiae anaphase-promoting complex interacts with multiple histone-modifying enzymes to regulate cell cycle progression. Eukaryot Cell 2010, 9, 1418–1431.
- Linger, J.G.; Tyler, J.K. Chromatin disassembly and reassembly during DNA repair. Mutat. Res. 2007, 618, 52–64.
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708.
- Kim, J.A.; Haber, J.E. Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete. Proc. Natl. Acad. Sci. USA 2009, 106, 1151–1156.
- Rodriges Blanko, E.; Kadyrova, L.Y.; Kadyrov, F.A. DNA mismatch repair interacts with CAF-1- and ASF1A-H3-H4-dependent histone (H3-H4)2 tetramer deposition. J. Biol. Chem. 2016, 291, 9203–9217.
- Gates, L.A.; Shi, J.; Rohira, A.D.; Feng, Q.; Zhu, B.; Bedford, M.T.; Sagum, C.A.; Jung, S.Y.; Qin, J.; Tsai, M.J.; et al. Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. J. Biol. Chem. 2017, 292, 14456–14472.
- Farooq, Z.; Banday, S.; Pandita, T.K.; Altaf, M. The many faces of histone H3K79 methylation. Mutat. Res. Rev. Mutat. Res. 2016, 768, 46–52.
- Espinosa, M.C.; Rehman, M.A.; Chisamore-Robert, P.; Jeffery, D.; Yankulov, K. GCN5 is a positive regulator of origins of DNA replication in Saccharomyces cerevisiae. PLoS ONE 2010, 5, e8964.
- Ichim, G.; Mola, M.; Finkbeiner, M.G.; Cros, M.P.; Herceg, Z.; Hernandez-Vargas, H. The histone acetyltransferase component TRRAP is targeted for destruction during the cell cycle. Oncogene 2014, 33, 181–192.
- Sinclair, D.A.; Guarente, L. Extrachromosomal rDNA circles—A cause of aging in yeast. Cell 1997, 91, 1033–1042.
- Defossez, P.A.; Prusty, R.; Kaeberlein, M.; Lin, S.J.; Ferrigno, P.; Silver, P.A.; Keil, R.L.; Guarente, L. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol. Cell 1999, 3, 447–455.
- Sullivan, M.; Holt, L.; Morgan, D.O. Cyclin-specific control of ribosomal DNA segregation. Mol. Cell Biol. 2008, 28, 5328–5336.
- Dubey, R.N.; Nakwal, N.; Bisht, K.K.; Saini, A.; Haldar, S.; Singh, J. Interaction of APC/C-E3 ligase with Swi6/HP1 and Clr4/Suv39 in heterochromatin assembly in fission yeast. J. Biol. Chem. 2009, 284, 7165–7176.
- Menzel, J.; Malo, M.E.; Chan, C.; Prusinkiewicz, M.; Arnason, T.G.; Harkness, T.A. The anaphase promoting complex regulates yeast lifespan and rDNA stability by targeting Fob1 for degradation. Genetics 2014, 196, 693–709.
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? Elife 2021, 10, e62852.
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194.
- Haigis, M.C.; Yankner, B.A. The aging stress response. Mol. Cell 2010, 40, 333–344.
- Longo, V.D.; Fabrizio, P. Regulation of longevity and stress resistance: A molecular strategy conserved from yeast to humans? Cell Mol. Life Sci. 2002, 59, 903–908.
- Furukawa-Hibi, Y.; Kobayashi, Y.; Chen, C.; Motoyama, N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid. Redox Signal. 2005, 7, 752–760.
- Postnikoff, S.D.; Malo, M.E.; Wong, B.; Harkness, T.A. The yeast forkhead transcription factors fkh1 and fkh2 regulate lifespan and stress response together with the anaphase-promoting complex. PLoS Genet. 2012, 8, e1002583.
- Malo, M.E.; Postnikoff, S.D.; Arnason, T.G.; Harkness, T.A. Mitotic degradation of yeast Fkh1 by the anaphase promoting complex is required for normal longevity, genomic stability and stress resistance. Aging 2016, 8, 810–830.
- Park, H.J.; Costa, R.H.; Lau, L.F.; Tyner, A.L.; Raychaudhuri, P. Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase. Mol. Cell Biol. 2008, 28, 5162–5171.
- Simpson-Lavy, K.J.; Sajman, J.; Zenvirth, D.; Brandeis, M. APC/CCdh1 specific degradation of Hsl1 and Clb2 is required for proper stress responses of S. cerevisiae. Cell Cycle 2014, 8, 3006–3012.
- Ahlskog, J.K.; Bjork, J.K.; Elsing, A.N.; Aspelin, C.; Kallio, M.; Roos-Mattjus, P.; Sistonen, L. Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress. Mol. Cell Biol. 2010, 30, 5608–5620.
- Chen, M.; Gutierrez, G.J.; Ronai, Z.A. The anaphase-promoting complex or cyclosome supports cell survival in response to endoplasmic reticulum stress. PLoS ONE 2012, 7, e35520.
- Kowalski, J.R.; Dube, H.; Touroutine, D.; Rush, K.M.; Goodwin, P.R.; Carozza, M.; Didier, Z.; Francis, M.M.; Juo, P. The Anaphase-Promoting Complex (APC) ubiquitin ligase regulates GABA transmission at the C. elegans neuromuscular junction. Mol. Cell. Neurosci. 2014, 58, 62–75.
- Mahoney, T.R.; Luo, S.; Nonet, M.L. Analysis of synaptic transmission in Caenorhabditis elegans using an aldicarb-sensitivity assay. Nat. Protoc. 2006, 1, 1772–1777.
This entry is offline, you can click here to edit this entry!
