Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Cepharanthine is an active ingredient separated and extracted from Stephania cepharantha Hayata, a Menispermaceae plant. As a bisbenzylisoquinoline alkaloid, cepharanthine has various pharmacological properties, including antioxidant, anti-inflammatory, immunomodulatory, antitumoral, and antiviral effects. Following the emergence of coronavirus disease 2019 (COVID-19), cepharanthine has been found to have excellent anti-COVID-19 activity.
- cepharanthine
- coronavirus disease 2019
- antiviral
- novel formulations
1. Important Physicochemical Properties of Cepharanthine
Cepharanthine is a member of the bisbenzylisoquinoline cyclic alkaloid family [7]. It is also known as 12-O-methyl cepharanoline and is characterized by the presence of a double 1-benzylisoquinoline portion in its alkyl chain. It is mainly found in plants such as Stephania epigaea and S. cepharantha [9]. Its molecular formula is C37H38N2O6, and it is a generally white or yellow crystalline powder. In the chemical structure of cepharanthine, there are two head-to-head connected coclaurine units, assigning it an elliptical macrocyclic structure. This confers the unique chemical properties of ether solubility, optical activity, and the ability to reduce the mobility of various biofilms [5,7]. Cepharanthine is easily soluble in acidic aqueous solutions and some organic solvents, such as methanol, ethanol, and DMSO, but is hardly soluble in water. It is cationic and amphiphilic [10]. These specific physicochemical properties lead to its low bioavailability. Doses of 1–60 mg per day have been safely and effectively used to treat various conditions. For example, the oral dose for alopecia areata treatment is usually 1.5–6 mg of CEP per day. The dose for the treatment of radiation-induced leukopenia is usually 50–60 mg of CEP per day. The dose for the treatment of multiple myeloma is usually 30 mg of CEP per day [10,11]. CEP has a half-life of 31.3–36.9 h and achieves a steady state after five to six repeated doses of 100 mg/day, with a maximum of 9.6% residual drug after oral administration [12]. The time to reach the maximum serum concentration after a single oral dose of 10–60 mg in healthy men ranges from 1.1 to 2.5 h. After absorption in the body, CEP is extensively metabolized in the liver and distributed to various tissues [5].
2. Pharmacological Effects of Cepharanthine
2.1. Antiviral Effects
A previous study has indicated that cepharanthine has a potent and extensive antiviral activity against viruses such as SARS-CoV, MERS-CoV, and HIV-1 [13]. It has been demonstrated that in chronically infected U1 cells, cepharanthine inhibits TNF-α or phorbol 12-myristate 13-acetate-induced HIV-1 replication by suppressing NF-κB activation [14]. The anti-HIV-1 activity of cepharanthine is dependent on its ability to inhibit NF-κB, and it inhibits the entry of HIV-1 by reducing plasma membrane fluidity [15]. Additionally, cepharanthine can affect the replication of viruses, such as hepatitis B virus [16], herpes zoster virus [17], and T-lymphotropic virus type 1 [18]. The extensive antiviral activity of cepharanthine may originate from the inhibition of intracellular inflammatory cytokines and chemokines [7].
COVID-19 is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has a complex mechanism of action. The virus has been reported to affect several vital organs in the body, including the lungs, heart, brain, kidneys, gastrointestinal tract, blood, and immune system [19]. Several vaccines have been developed, but their availability is limited, and they can only have a preventive effect. To date, no specific therapeutic agent for coronavirus infection exists. Several drugs approved for COVID-19 by the US Food and Drug Administration (FDA) have previously been used for other diseases, such as raltegravir (Ebola) [20], lopinavir–ritonavir (AIDS) [21], chloroquine (malaria) [22], and favipiravir (influenza) [23]. In early 2020, Yigang et al. discovered that cepharanthine could be a potent drug for treating COVID-19 infection after screening thousands of monomeric compounds [24].
Subsequently, the anti-COVID-19 activity of cepharanthine has been confirmed repeatedly [25,26]. The combined use of nelfinavir and cepharanthine could effectively inhibit SARS-CoV-2 replication, wherein cepharanthine inhibits the entry of SARS-CoV-2 by blocking the binding of the virus to target cells, and nelfinavir inhibits viral replication by inhibiting proteases [27]. Moreover, cepharanthine significantly inhibited the purified recombinant SARS-CoV-2 decapping enzyme, Nsp13, via in vitro enzyme activity assays. Nsp13 is essential for viral replication and is the most conserved nonstructural protein in the coronavirus family [26]. RNA-seq analysis has revealed that cepharanthine may exert antiviral effects through the reversal of heat shock factor-1-mediated heat shock response, endoplasmic reticulum stress/unfolded protein response, and hypoxic pathways of viral interference [28]. A pseudoviral model of SARS-CoV-2 demonstrated that cepharanthine can inhibit SARS-CoV-2 S protein/angiotensin-converting enzyme 2 (ACE2)-mediated membrane fusion by targeting host calcium ion channels and simultaneously upregulating intracellular cholesterol levels, thereby effectively inhibiting infection by SARS-CoV-2 mutants and different coronaviruses [13]. The human coronavirus OC43 model was used to screen 1900 clinically used drugs in vitro, which revealed that cepharanthine had the highest in vitro anti-SARS-CoV-2 activity, much higher than that of the already marketed anti-SARS-CoV-2 drug, raltegravir [29].
2.2. Prevention of Leukopenia
Cepharanthine was first used to increase the number of leukocytes in the peripheral blood of patients undergoing radiotherapy or chemotherapy [7]. The major mechanisms involved are now widely recognized as the stimulation of the reticuloendothelial system, the activation of hematopoietic tissue, and the promotion of bone marrow proliferation, which ultimately increases the white blood cell count [30].
Leukopenia is clinically defined as a reduction in the number of neutrophils and can be caused by many factors, including chemotherapy and radiotherapy for tumors and chemicals such as benzene. In the 1930s and 1940s, cepharanthine was used to treat pulmonary tuberculosis. It was found that patients with tuberculosis had an increased number of leukocytes in the peripheral blood after cepharanthine administration, which indicated the prospect of cepharanthine in preventing leukopenia. In several clinical studies involving more than 350 patients, radiotherapy was used along with cepharanthine to treat head and neck tumors and ovarian, lung [10], and breast cancers [31]. All studies showed that cepharanthine helped prevent leukopenia in patients receiving anticancer treatment.
2.3. Antitumor Effects
Cepharanthine exhibits antitumor effects by enhancing immunity [32] and inhibiting tumor cell proliferation [33], as well as increasing the sensitivity of tumor cells to radiotherapy while reducing the adverse effects caused by the radiotherapy [34]. A more promising approach is the use of cepharanthine in combination with other chemotherapeutic drugs to reverse multidrug resistance (MDR) of tumor cells [35]. CEP effectively inhibits the drug transporter protein ABCC10 (also known as MRP7) on cancer cell membranes, thereby reversing anticancer drug resistance in cells expressing ABCC10. This allows for the increased accumulation of anticancer drugs in cancer cells and inhibits their efflux [36], thus improving the efficacy of anticancer drugs. CEP also interferes with other transporter proteins, such as ABCB1 (also known as MDR1 or P-glycoprotein) [37], possibly by inhibiting the PI3K/Akt signaling pathway, leading to the downregulation of ABCB1 expression in cancer cells, which in turn reverses MDR [38]. CEP potentially interacts directly with specific drug transporter proteins present on the plasma membrane. For example, neferine, another bisbenzylisoquinoline alkaloid with a similar structure to CEP, binds strongly to and inhibits ABCB1, thereby reversing MDR. Therefore, it is speculated that CEP has a similar biological effect [39].
Cepharanthine can be involved in tumor prevention and treatment through various mechanisms, including increasing the white blood cell count and exerting immunomodulatory effects on macrophages, T cells, and natural killer cells to increase the immune capacity [32,40]. Additionally, it can play a role in inducing apoptosis, inhibiting tumor cell infiltration and metastasis.
2.4. Anti-Inflammatory Effects
Cepharanthine is also effective for inflammation in vivo, especially in the ears, nose, throat, and mouth. It can significantly decrease the expression of certain inflammatory cytokines, such as IL-6, IL-1β, and TNF-α [41]. In a mouse model of lipopolysaccharide (LPS)-induced mastitis, cepharanthine significantly reduced neutrophil infiltration and decreased TNF-α, IL-1β, and IL-6 levels [41]. Presently, cepharanthine is used to treat inflammatory diseases such as rheumatism, arthritis, lumbago, nephritis, edema, and dysentery [7].
2.5. Immunomodulation
Cepharanthine can be used as an immunomodulator, and it has excellent potential in the treatment of various autoimmune diseases and allergies [42]. At a low dose, cepharanthine could effectively prevent progressive thrombocytopenia and was used to successfully treat a patient with multiple myeloma combined with immune thrombocytopenic purpura [43]. Additionally, cepharanthine specifically inhibits abnormally activated T cells or steroid-resistant human leukemia T cells [44,45]. Furthermore, cepharanthine regulates several signaling pathways in abnormally activated T cells, such as NF-κB, caspase cascade, cell cycle, mitogen-activated protein kinase (MAPK), and PI3K/Akt/mTOR pathways [46].
3. Molecular Mechanisms of Cepharanthine
3.1. NF-κB Pathway
The NF-κB pathway plays a central role in inducing pro-inflammatory gene expression and is a potent regulator of inflammatory molecules. In resting cells, NF-κB exists in the cytoplasm as a latent, inactive IκB-binding complex. Upon relevant stimulation, IκB kinase (IKK) induces phosphorylation of the IκB protein via the ubiquitin–proteasome pathway, resulting in its rapid degradation, allowing NF-κB dimers to enter the nucleus and activating specific target gene expression [46]. In an LPS-stimulated model of RAW264.7 cells, cepharanthine inhibited NF-κB activation by blocking the IKK pathway [47] (Figure 2). In this study, cepharanthine (2.5, 5, and 10 μg/mL) inhibited the release of pro-inflammatory factors such as TNFα, IL-6, and IL-1β in a dose-dependent manner with potentially no cytotoxicity. In addition, 10 mg/kg/day of cepharanthine could significantly inhibit the expression of NF-κB and reduce the levels of the pro-inflammatory cytokines, IL-1β and TNF-α, in diabetic rats [48]. Thus, it seems that cepharanthine can reduce the expression of inflammatory factors in related disease models through the NF-κB pathway and exert anti-inflammatory effects as a potential drug for the treatment of certain related diseases.
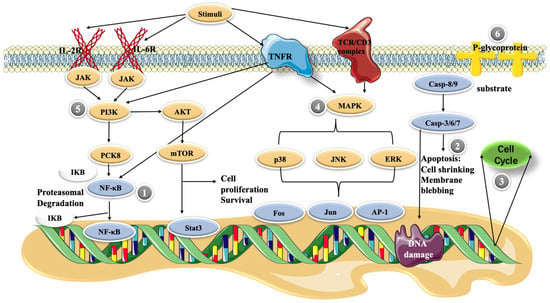
Figure 2. Possible molecular mechanisms of cepharanthine in cells. (1) NF-κB pathway, (2) apoptosis, (3) cell cycle control, (4) MAPK pathway, (5) PI3K/Akt/mTOR signaling pathway, and (6) P-glycoprotein expression.
3.2. Apoptosis
Apoptosis is a regulated cellular suicide mechanism characterized by nuclear condensation, cell shrinkage, membrane leakage, and DNA fragmentation [49]. The cascade of caspases, which belong to the cysteine protease family, is a key apoptosis regulator. To date, only two types of caspases have been defined, including initiator and effector caspases. Initiator caspases, such as caspase-2, -4, -8, -9, -10, and -12, are closely associated with proapoptotic signaling. Upon stimulation by apoptosis, these initiator caspases divide and activate downstream effector caspases, such as caspase-3, -6, and -7. These effector caspases hydrolyze a range of substrates, generating signals that lead to cell death [50,51]. The cell growth inhibition of primary effusion lymphoma (PEL) cell lines was enhanced in a dose-dependent manner as the cepharanthine dose was increased from 1 to 10 μg/mL. Moreover, cepharanthine-treated PEL cells induced the activation of caspase-3, which is an apoptosis marker [52]. In addition, cepharanthine at high concentrations (10–20 μg/mL) decreased the mitochondrial membrane potential of dendritic cells, and high levels of cellular stress caused the release of cytochrome c from mitochondria. This activates caspase-9, which then activates the effector caspase-3/7, and finally triggers apoptosis [53].
3.3. Cell Cycle Control
The cell cycle is regulated by different cellular proteins, such as the cell cycle protein A/B/D [54]. It has been shown that cepharanthine affects the cell cycle, usually arresting cells in the G1 and S phases. Jurkat T cells treated with 5, 10, and 15 μΜ CEP showed a dose-dependent inhibition of cell cycle progression in the S phase, significantly reducing the number of cells in the G0/G1 phase [45]. Further research has revealed that cepharanthine upregulates the expression of cell cycle proteins A2 and B1 but downregulates that of the cell cycle protein D1 in Jurkat T cells, possibly relating to cell cycle arrest [46]. Using 0, 1, 5, 10, and 20 μM cepharanthine also effectively reverses MDR-mediated resistance to cisplatin in esophageal squamous cell carcinoma (ESCC) cells, inhibiting the proliferation of ESCC cell lines and inducing G2/M phase cell cycle arrest. Dose-dependent upregulation of p53 and downstream p21 induced by cepharanthine explains its mechanism of causing cell cycle arrest [55].
3.4. MAPK Pathway
Activated MAPK has been reported to play an important role in promoting and maintaining T lymphocyte populations [56]. Cepharanthine regulates multiple signaling pathways that aberrantly activate T cells at low toxicity, and the antiproliferative effect on human peripheral blood T cells occurs partly through the blockade of MAPKs, such as JNK, p38, and ERK activity [57]. It was reported that another class of bisbenzylisoquinoline alkaloids, tetrandrine, in combination with methylprednisolone (a glucocorticoid), significantly inhibited the phosphorylation of the mitogen-activated protein kinase family. Powdered tetrandrine (3 μM) in combination with 0.5 ng/mL methylprednisolone showed synergistic inhibition of both ERK1/2 and P38. The powdered antifungal base significantly reduced the IC50 value of methylprednisolone but had no significant toxic effect on normal cells [46]. These evaluations suggest that CEP, a member of the bisbenzylisoquinoline alkaloid family, may have similar efficacy and could be used as a lead compound for the development of new drugs for the treatment of T-cell-related diseases or to address glucocorticoid resistance.
3.5. PI3K/Akt/mTOR Signaling Pathway
The PI3K/Akt/mTOR signaling pathway is involved in regulating various cellular responses, such as metabolic regulation [58], cell proliferation, transcription, translation, survival, and growth [58,59]. This pathway is essential for the pathological and physiological conditioning of humans, and changes in the regulation of this pathway may lead to the development of various cancers [60]. Thus, the PI3K/Akt/mTOR signaling pathway is a potential target for antitumor therapy. In a study on breast cancer cells, treatment with 5 and 10 μM cepharanthine was found to reduce the levels of both phosphorylated AKT and mTOR. The downstream targets of mTOR (p70S6K, p-S6, and p-4E-BP1) were significantly reduced, whereas GSK3β, a downstream effector of AKT [61], showed similarly reduced levels in cells after CEP treatment. These results suggest that cepharanthine induces apoptosis and autophagy in breast cancer cells by inhibiting the AKT/mTOR signaling pathway[62]. Cepharanthine can inhibit the expression of p-PI3K and mTOR in Jurkat T cells; however, this resulted in high expression of p-Akt1 [45]. This result may appear to be incongruous, as Akt is considered a major downstream effector of PI3K in physiological processes [63]. However, several studies have demonstrated that different groups of tyrosine (Ack1/TNK2, Src, and PTK6) and serine/threonine (TBK1, IKBKE, and DNAPKcs) kinases can directly activate Akt [64]. Thus, CEP may regulate p-PI3K and mTOR expression independently of Akt.
3.6. P-glycoprotein Expression
P-glycoprotein (also known as ABCB1) belongs to the ABC superfamily of transporter proteins [65] and is encoded by multidrug resistance gene 1 (MDR-1), which is an ATP-dependent membrane transporter protein [66]. The overexpression of drug transporter proteins present in cancer cell membranes is a major cause of MDR to cancer chemotherapy. Cepharanthine can reverse the resistance of tumor cells to many chemotherapy drugs by interfering with P-glycoprotein [37]. Cepharanthine may act as a potent P-glycoprotein inhibitor, dose-dependently restoring antitumor activity [67]. In a study on glucocorticoid resistance, the human T-lymphoblast leukemia cell line MOLT-4 with low P-glycoprotein expression, and its MDR subline MOLT-4/DNR, were used as the model. Tetrandrine (Figure 3), being likewise a bisbenzylisoquinoline-like alkaloid and highly resembling cepharanthine, indirectly modulated the translocation of glucocorticoid receptors by inhibiting the efflux function of P-glycoprotein in MOLT-4/DNR cells and downregulated its protein expression, thereby enhancing the pharmacodynamic effects of glucocorticoids. The expression of P-glycoprotein was downregulated in MOLT-4/DNR cells in a concentration-dependent manner under treatment with 0.03, 0.3, and 1 μM tetrandrine. Although 1 μM tetrandrine significantly inhibited P-glycoprotein expression, MOL T-4/DNR 296 cells exhibited cytotoxic effects at this dose. In contrast, glucocorticoid receptor translocation was almost completely restored at 0.3 μM with no notable side effects [68]. However, studies on the resistance of CEP to glucocorticoids remain unclear, and the actual effective dose needs to be explored further. However, it can be deduced that this bisbenzylisoquinoline alkaloid of natural plant origin is beneficial for glucocorticoid-resistant diseases caused by P-glycoprotein overexpression.
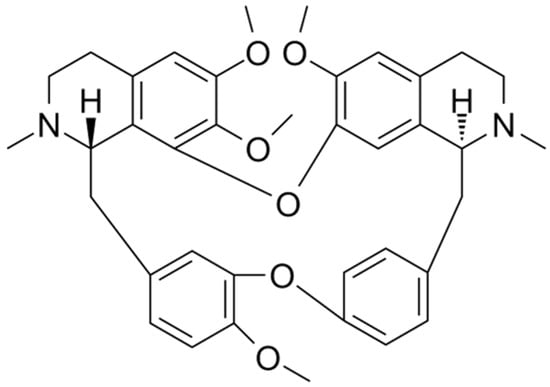
Figure 3. Chemical structure of tetrandrine.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27248933
This entry is offline, you can click here to edit this entry!
