Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The ATP-binding cassette transporters (ABC transporters) are a large family of proteins that transport a variety of substrates across cell plasma membranes. Because of this, they are involved in many physiological processes. It is of interest to note that many ABC transporters are involved in the transport of various lipids. In addition, this function may be related to the innate immune system.
- ABC transporters
- lipids
- innate immune system
- ABCA1
- ABCB1
- lipopolysaccharide
1. Introduction
The ATP-binding cassette transporters (ABC transporters) are a family of proteins that transport chemically different substrates through the lipid bilayer of cell membranes, at the expense of the energy obtained by ATP hydrolysis. Currently, 48 human ABC transporters have been described, which are divided into seven subfamilies, known as ABCA to ABCG, based on their structural organization. ABC transporters contain two conserved nucleotide-binding domains (NBD), which convert the energy of ATP binding and hydrolysis into conformational changes of two transmembrane domains (TMD) that are involved in substrate recognition [1][2].
Due to the chemical diversity of substrates, ABC transporters are widely involved in various biological processes [3]. Members of the ABCA and ABCG subfamilies are well known for their role in the transport of various lipids, which allows them to be considered as important players in many clinically relevant diseases, such as atherosclerosis. In turn, members of the ABCG and ABCC subfamilies are involved in the mechanisms of multidrug resistance, which is of great clinical importance and is the subject of numerous studies.
The export of substrates by ABC transporters is described through the “alternating access” model, which involves switching between inward and outward facing transmembrane cavity (Figure 1) [4]. NBDs dimerize and induce a switch of TMDs from an inward-facing conformation to an outward-facing conformation, thereby moving the substrate [5]. In turn, TMDs form a transport substrate-binding cavity, which can open into either the cytoplasmic or exofacial leaflet of the plasma membranes [6].
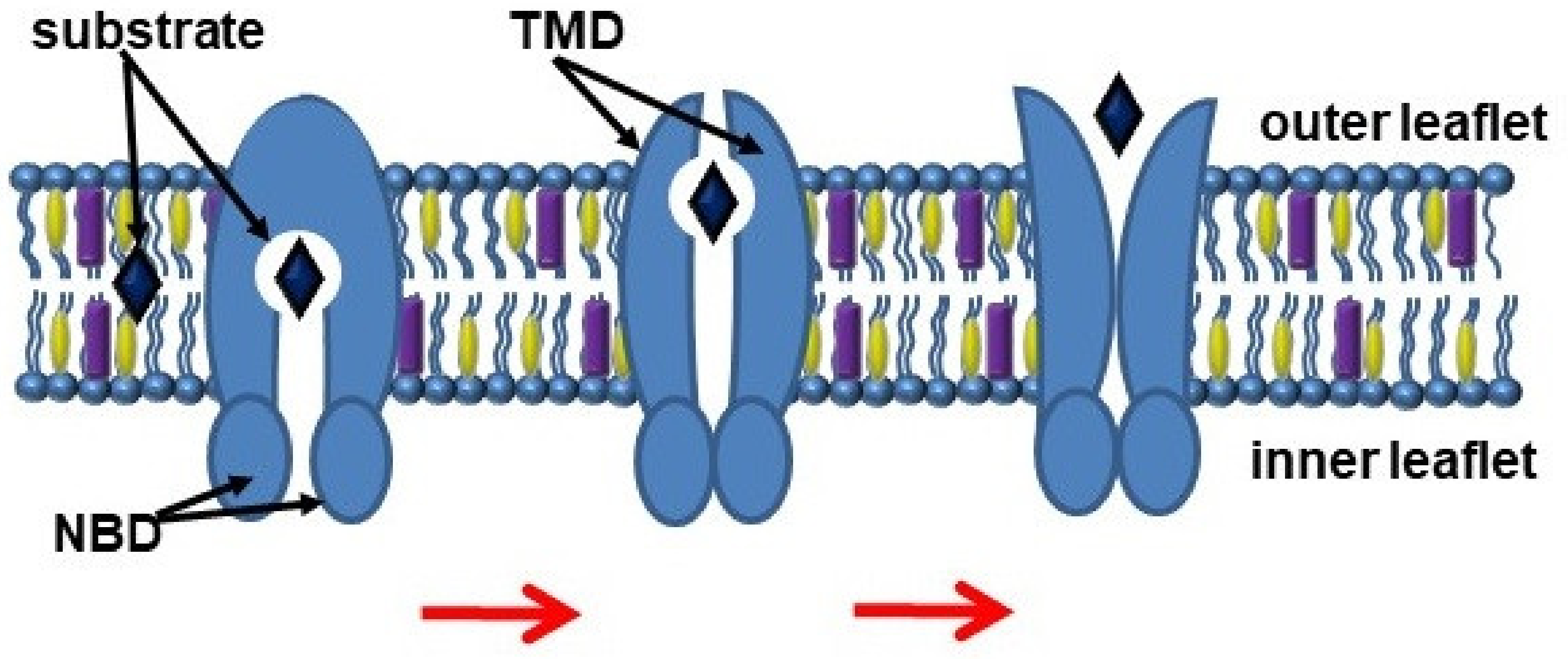
Figure 1. Scheme of the ABC transporter function. Abbreviations: NBD, nucleotide-binding domains; TMD, transmembrane domains.
2. ABC Transporters
2.1. The ABCA Subfamily
The ABCA subfamily consists of 12 members which are involved in the transport of lipid molecules across membranes [7]. Members of this subfamily are well known for their role in lipid transport [3]. ABCA1 is considered to be a key participant in reverse cholesterol transport, a process by which cholesterol moves from cells to the extracellular acceptor to form the resulting HDLs (Figure 2). Macrophages, as a result of reverse cholesterol transport, maintain an optimal balance of cholesterol, the excessive accumulation of which has proinflammatory effects. Absence of ABCA1 leads to significant changes in the morphology, properties, and functional activity of macrophages [8]. In addition to free (non-esterified) cholesterol transport, ABCA1 functions as a membrane phospholipid translocase, and its enzymatic activity leads to the transfer of phospholipid molecules from the cytoplasmic leaflet to the outer leaflet of the cellular plasma membrane [6].
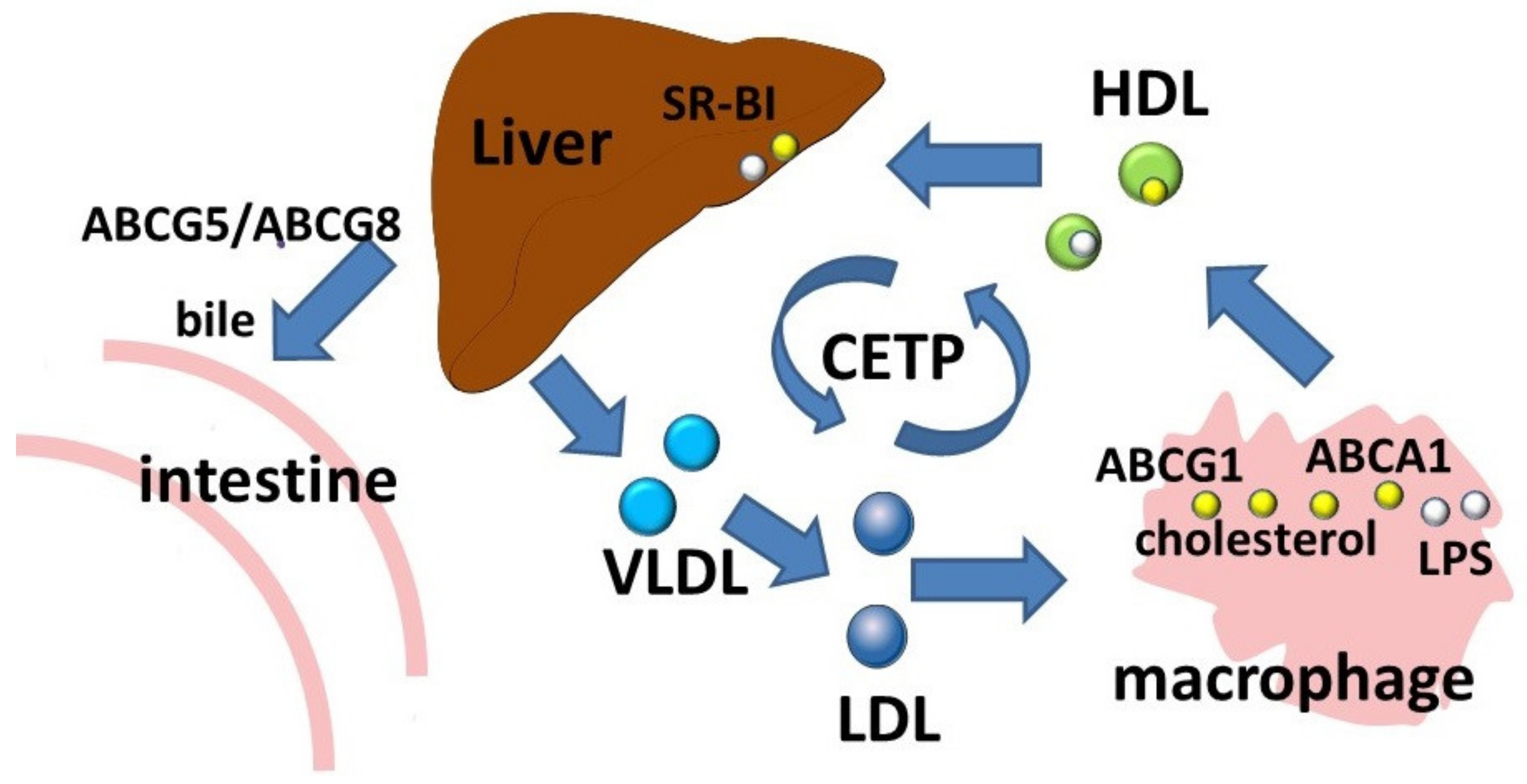
Figure 2. Reverse cholesterol transport and LPS transport. Abbreviations: ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette subfamily G member 1; CETP, cholesteryl ester transfer protein; HDL, high density lipoprotein; LDL, low density lipoprotein; LPS, lipopolysaccharide; SR-BI, scavenger receptor class B type 1; VLDL, very low density lipoprotein.
ABCA1 has been shown to exert an anti-inflammatory effect by promoting cholesterol efflux followed by attenuation of signal transduction via Toll-like receptors [9]. At the same time, macrophages Abca1−/− Abcg1−/− have shown enhanced inflammatory gene responses to TLR2, TLR3, and TLR4 ligands [9][10]. In turn, Abca1−/− and Abcg1−/− macrophages were shown to have increased lipid rafts and produced higher levels of tumor necrosis factor alpha (TNFα) and Interleukin (IL)-6 after LPS stimulation compared with wild-type macrophages [9]. Thus, the involvement of ABCA1 in TLR4 regulation may be through changes in cholesterol content in the lipid rafts of the plasma membrane, into which signaling molecules such as TLR4 are recruited.
ABCA1 predominantly localizes in the plasma membrane of the cell, which requires palmitoylation of the protein [11][12][13][14]. ABCA1 activity leads to the reorganization of lipids in the plasma membrane, which is characterized by redistribution of membrane cholesterol from raft domains to non-raft domains [15][16]. At the same time, ABCA1 itself can be located either in the non-raft domains [16][17][18] or in the raft domains [6][19]. Interestingly, ceramide enhances cholesterol efflux into apolipoprotein A-I by increasing the presence of ABCA1 on the cell surface [20].
Cholesterol is involved in maintaining the spatial structure of the plasma membrane. This is related to the chemical structure of the cholesterol molecule and its spatial arrangement in the plasma membrane. Cholesterol is an important component of lipid rafts, and changes in cholesterol content in the plasma membrane affect its structure and function. Cholesterol can influence the biophysical properties of the membrane and can also interact directly with specific protein sites, so that it can participate in the regulation of the function of membrane proteins [21].
Proteins that interact with cholesterol are thought to have specific amino acid sequences that play a role in this interaction [22]. A known amino acid sequence is the cholesterol-binding domain (CRAC, Cholesterol Recognition/interaction Amino acid Consensus sequence), which has been identified in proteins that interact with or are regulated by cholesterol [23][24][25][26]. The CRAC amino acid sequence includes the following set of amino acids: L/V–X (1–5) –Y–X (1–5) –R/K (with X = any amino acid) [25]. The other sequence, the CARC motif, has similar properties in binding to transmembrane proteins and has the reverse amino acid sequence: R/K–X (1–5) –Y/F–X (1–5) –L/V, (with X = any amino acid and tyrosine can be replaced with phenylalanine). Both CRAC and CARC sequences have been found in the transmembrane domain of the TLR4 receptor, providing a putative link between cholesterol and the regulation of receptor signal transduction [22].
ABCA1 can alter the cholesterol content of macrophage plasma membranes, which affects the stability of lipid rafts and can regulate TLR4 activity [27]. Deletion of ABCA1 leads to increased localization of TLR4 in lipid rafts in both resting and stimulated states [27]. Abca1-deficient mice have increased circulating levels of chemokines, cytokines, and growth factors. In addition, Abca1-deficient macrophages contain more cellular cholesterol ester, which corresponds to higher expression of scavenger receptors and greater TNFα secretion in response to LPS [8]. In turn, ABCA1 overexpression leads to a significant redistribution of cholesterol and sphingomyelin from lipid rafts to non-raft membrane regions, which can degrade membrane lipid rafts and enhance cholesterol efflux into ApoA-I [15].
In turn, TLR4 activation can inhibit ABCA1 expression, reducing the macrophage cholesterol efflux [28][29].
Decreased transport function of ABCA1 leads to excessive intracellular accumulation of cholesterol in macrophages, which contributes to the initiation of NLRP3 inflammation. Cholesterol accumulation in Abca1/Abcg1-deficient myeloid cells has been shown to activate the NLRP3 inflammasome, which promotes neutrophil infiltration and netosis in atherosclerotic lesions [30].
The ABCA1 transporter has also been shown to be involved in the removal of an immunostimulatory bacterial lipid, LPS (Figure 2) [31][32]. In experiments with preconditioning of ABCA1-deficient macrophages with LPS, a significant decrease in LPS outflow was observed, resulting in prolonged persistence of LPS on the cell surface [32].
ABCA1 is an important subject for studies on the pathogenesis of atherosclerosis. ABCA1 mutations are associated with the development of Tangier disease, which is characterized by a significant decrease in HDL levels, which corresponds to premature atherosclerosis [33]. Decreased ABCA1 protein has been shown to play a key role in the pathogenesis of carotid atherosclerosis [34]. In one study, ABCA1 protein expression was significantly decreased in plaques compared with control tissues [35]. Moreover, ABCA1 may serve as an important marker of plaque instability [34]. Leukocyte ABCA1 has been shown to play an important role in protecting against atherosclerosis, whereas more widespread and larger atherosclerosis lesions develop in the absence of ABCA1 leukocyte [36]. These and other data suggest that ABCA1 is a promising target for drug action to prevent and treat atherosclerosis [37].
Another member of the subfamily, ABCA7 is involved in cross-links between fatty acid metabolism and inflammation in the brain. Disturbances in these connections may contribute to the development of Alzheimer’s disease [38]. In addition, several studies have shown possible involvement of ABCA7 in phagocytosis [39][40]. ABCA7 is expressed in macrophages [39][41][42], where it can be localized in the plasma membrane, or intracellularly [43][44][45][46]. The putative involvement of ABCA7 in phagocytosis is due to the fact that the protein has high amino acid sequence similarity with CED-7, which is required for efficient phagocytosis of apoptotic cells [40]. The CED-7 protein in the nematode Caenorhabditis elegans functions in phagocytic and apoptotic cells during phagocytosis and is required for the clustering of CED-1, a transmembrane receptor that initiates uptake signals. In doing so, CED-7 carries out the exposure of phospholipid ligands on the surface of apoptotic cells. The ABCA5 protein is also involved in cholesterol transport, and ABCA5 expression has been shown to increase in monocytes and macrophages after incubation with acetylated LDL [47][48].
Thus, members of the ABCA subfamily play a significant role in the mechanisms of innate immunity mediated by lipid transport, which is of great clinical importance [49][50].
2.2. The ABCB Subfamily
ABCB1 is the most well-characterized member of the ABCB subfamily. The ABCB1 (MDR1, P-glycoprotein) protein encoded by the ABCB1 gene is expressed by various immune cells where it may be involved in migration, differentiation, survival, or cytotoxic function [51].
This transporter is well known for its role in multidrug resistance due to its ability to efflux a variety of molecules with different chemical structures (cyclic, linear, polar, non-polar, linear-hydrophobic, aromatic) and different molecular weights (from 250 to 4000 Da) [52]. ABCB1 has a wide substrate specificity, which allows it to transport chemically diverse molecules, including drugs [53][54][55].
ABCB1 has been shown to transport lipophilic and amphipathic compounds that accumulate in the lipid bilayer, lining up in the interphase region between the lipid head group and the first few carbon atoms of the lipid acyl chains [56]. Given the knowledge that ABCB1 removes drugs directly from the membrane rather than from the aqueous phase [54], it is assumed that the transporter is a hydrophobic “vacuum cleaner” responsible for removing potentially harmful lipophilic compounds from the membrane [52][57].
The capture of ABCB1 drugs inside the lipid membrane can occur due to weak electrostatic interactions between hydrogen bond acceptor groups, including phenyl rings and tryptophans (i.e., π-electron donor systems), in drugs and hydrogen bond donor groups (i.e., π-electron acceptor systems) in the transmembrane region of ABCB1 [58]. Whether a drug can be classified as a substrate for ABCB1 depends on the cross-sectional area of the drug as well as on the lipid composition of the plasma membrane, which determines the lateral density of lipid packing [59][60]. It is important to note that lipid packing density is affected by membrane composition, including cholesterol content [58].
In addition to xenobiotic transport, ABCB1 performs many other physiologically relevant functions, such as being involved in lipid transport [53][57][61], moving lipids from the inside to the outside of the cell plasma membrane [22][53]. Cholesterol is also transported by ABCB1 [62]. Interestingly, the cholesterol content in the plasma membrane can regulate the functional activity of the transporter [63][64][65][66]. This may be due to the direct interaction of ABCB1 with lipid molecules [22][53][67].
Links between ABCB1 and innate immunity support the information that TLR4 ligands and inhibitors modulate ABCB1 activity, suggesting that interaction with TLR4 is important for ABCB1 function. The innate immune inflammatory response may play a role in the ocular trabecular meshwork through modulation of ABCB1. The functional activity of ABCB1 is influenced by TLR4 agonists, suggesting that TLR4 modulation is important for ABCB1 function [68].
ABCB1 and TLR show partially overlapping substrate specificity, as TLRs can recognize some substances that have the same recognition patterns as the allocrites for ABCB1 [58]. Meanwhile, ABCB1 binds cationic and electrically neutral compounds, including nucleosides, whereas PRRs rather recognize anionic nucleotides and are activated by bacterial lipid A surrounded by anionic phosphate groups [58].
TLR2 is thought to act as a central regulator of xenobiotic defense through ABCB1. TLR2 has been shown to modulate the synthesis and activity of ABCB1 in mouse and human myeloid cells by stimulating xenobiotic efflux, which reduces cytotoxicity. Activation of ABCB1 transcription involves the MKK3/6 → p38 MAPK pathway [69], which is common to TLR2 signaling [70]. This mechanism allows the innate immune system to respond to harmful substances and carry out their immediate elimination [71].
ABC/MXR transporters are known to play a role in LPS efflux [72]. It has been shown that LPS uptake by the cells of the gastrointestinal tract can be excreted by ABCB1. Abcb4 efflux of LPS in fish back into the intestinal lumen may represent a protective mechanism to limit systemic LPS uptake and inflammation [72].
In addition to these mechanisms, ABCB1 has other extensive connections to the immune system. ABCB1 has been shown to be important for the secretion of proinflammatory cytokines such as TNFα and IFN-gamma [73]. ABCB1 is thought to mediate dendritic cell maturation and T-cell responses by influencing TNFα and IFN-γ production, thereby regulating immune responses [73]. Interestingly, ABCB1 is important for the complete activation of the response to type I interferon induced by Listeria monocytogenes bacteria. Moreover, inhibition of ABCB1 function by verapamil or inhibition of its transcription using mRNA silencing reduced the magnitude of the type I response in infected cells, i.e., decreased IFN release, which shows the importance of ABCB1 for the proper development of the innate immune response against intracellular pathogens [74]. In addition, R(+) verapamil improved the survival of mice that received a lethal dose of LPS by inhibiting ABCB1, which was accompanied by decreased TNFα and IFN-gamma levels and higher levels of IL-6 [75].
In addition to IFN-gamma and TNFα, ABCB1, may be involved in the release of cytokines such as IL-2 and IL-4. In addition, ABCB1 is involved in IL-12-dependent differentiation of monocytes in the dendritic cell line during antigen-presenting cell maturation. Because of this, ABCB1 regulates the ability of myeloid-derived antigen-presenting cells to elicit alloimmune Th1 responses [76]. Specific ABCB1 blockade inhibits IL-12 secretion and activation of human alloimmune T cells in vitro [77]. ABCB1 is involved in dendritic cell maturation when exposed to LPS and hypoxia, whereas inhibition of ABCB1 impaired dendritic cell maturation and reduced alloimmune T-cell proliferation [78]. ABCB1 is also involved in the regulation of leukocyte movement, especially of dendritic cells from tissues through lymphatic channels [79]. Given that dendritic cells are the link between innate and adaptive immunological responses, the importance of ABCB1 is of increasing interest.
ABCB1 is also known to secrete platelet-activating factor (PAF) [80]. PAF is a lipid mediator that mediates inflammation and is produced in response to various stimuli, including lipopolysaccharide, tumor necrosis factor, interleukin-1, activated complement, and angiotensin II [81].
Overall, these data suggest an important role for ABCB1 in innate immunity [58]. At the same time, ABCB1 inhibitors such as verapamil can impair the regulation of the immune response. This is supported by reports of the development of colitis in mdr1a-deficient mice. The intestinal inflammation seen in mdr1a−/− mice results from a defect in the intestinal epithelial barrier and is characterized by dysregulation of epithelial cell growth and leukocytic infiltration into the lamina propria of the large intestine, and is consistent with signs of ulcerative colitis in humans [82]. Interestingly, colitis in these animals can be prevented by oral treatment with broad-spectrum antibiotics to kill intestinal bacteria. Even after the inflammation has ceased, a large number of CD3+ T cells remain in the intestinal mucosa [82].
Subsequent studies have greatly expanded the understanding of the role of ABCB1 in gut immunology. The gut microbiome is known to contribute to gut health and is also involved in the regulation of other organs, such as the lungs, through the production of some biologically active substances. The gut microbiome has evolved with the host, so the structure of the gut microbiome is closely related to dietary patterns, which is of great clinical importance. The gut microbiome and the ABCB1 of the gut epithelium have been found to be jointly involved in maintaining gut health. Bacterial populations containing the classes Bacilli and Clostridia contribute to the induction of ABCB1 expression in the colon by acting through butyrate and secondary bile acids produced by these bacteria from food substrates [83][84]. Butyrate, which is a short-chain fatty acid (SCFA), increases ABCB1 expression in colonic epithelial cell lines [83][85]. In patients with ulcerative colitis, there is a decrease in the number of butyrate-producing bacteria such as Roseburia hominis and Faecalibacterium prausnitzii and a decrease in the concentration of secondary bile acids in the intestine [83][86][87][88].
In addition, lithocholic acid (LCA), deoxycholic acid (DCA), and ursodeoxycholic acid (UDCA), secondary bile acids produced by intestinal bacteria, including representatives of Clostridia and Bacilli, by deconjugation and conversion of primary bile acids, enhance the induction of ABCB1 protein expression [84][89]. Thus, the gut microbiome and diet play an important role in maintaining gut health through ABCB1. These findings are supported by evidence that colitis was preceded by altered gut bacterial composition, and a high-fat diet increased the frequency and severity of colitis in Abcb1 KO mice without specific pathogens [90][91].
Lower levels of ABCB1 in the colon have been shown in patients with active inflammation and ulcerative colitis compared with controls [90][92][93]. Moreover, low levels of ABCB1 in the colon may precede malignancy [94]. Thus, ABCB1, diet, and intestinal bacteria mutually interact in colonic inflammation, which is of great clinical importance [90].
Another function of ABCB1 in gut immunology is related to the regulation of neutrophil infiltration. ABCC2 (MRP2) has been found to regulate transepithelial neutrophil migration by apical release of hepoxylin A3 (HXA3), which is a potent chemoattractant [95]. At the same time, intestinal ABCB1 exports N-acyl ethanolamine-type (NAE) endocannabinoids (eCB), which can inhibit this transepithelial migration through eCB interaction with neutrophilic cannabinoid receptor 2 (CB2), which is an important mechanism for the homeostasis of neutrophil function regulation in the gut [83][89].
Information on the protective role of ABCB1, which is expressed on intestinal epithelial cells against Listeria monocytogenes, is of interest [96][97]. Caco-2/MDR cells overexpressing ABCB1 have been shown to be characterized by resistance to L. monocytogenes invasion, whereas inhibition of ABCB1 resulted in increased invasion [96]. Meanwhile, Salmonella enterica serovar Typhimurium modulates, which is a facultative intracellular pathogen, specifically suppresses ABCB1 function, thereby increasing its ability to invade [98]. It has been found that the effector S. Typhimurium effector protein, SipA, modulates ABCB1 activity through a pathway involving caspase-3 [99]. These properties of Salmonella enterica are of great clinical interest as one of the promising areas of anti-tumor therapy [100]. Pseudomonas aeruginosa secreted the toxin Cif (Cystic fibrosis transmembrane conductance regulator (CFTR) inhibitory factor), which selectively reduces the expression of ABCB1 on the apical membrane in renal, respiratory, and intestinal epithelial cells [101]. These and other data confirm the protective role of the ABCB1 transporter in protecting the host not only against xenobiotics, but also against bacterial pathogens.
It is of interest to know that ABCB1 expression is a distinctive feature of mature naive B-cells because it increases during final differentiation from transient B-cells and is irreversibly lost by all memory B-cells [102]. At the same time, ABCB1 expression can affect the migration ability and location of naive B-cells relative to transient B-cells and memory B-cells, as well as influence the structure and function of lipid rafts, which are crucial for B-cell signaling. Lipid rafts act as platforms in B cells for B-cell receptor (BCR) signaling and antigen targeting, and the association of BCRs with lipid rafts changes during B-cell development [103].
In addition, ABCB1 can participate in the pathogenesis of various skin diseases [104]. A protective role of these transporters has been shown in a model of dermatitis in Mdr1a/1b/Bcrp−/− mice, as lesions in Mdr1a/1b/Bcrp−/− mice were more severe [105]. On the other hand, ABCB1 inhibitors are considered as a candidate for a possible therapeutic agent for the treatment of acne. Propionibacterium acnes has been shown to promote sebum secretion due to ABCB1 activation simultaneously with increased ABCB1 expression, which may be the result of TLR2 pathway activation in differentiated hamster sebocytes (DHS) [106]. Uveal melanoma cells expressing ABCB1 have also been shown to have a greater potential for metastasis, while exhibiting significantly increased mitochondrial activity compared with ABCB1−/− [107].
Numerous studies have also demonstrated the importance of ABCB1 in the pathogenesis of Alzheimers disease [108][109][110][111][112][113]. Amyloid beta (Aβ) deposition in Alzheimer’s disease has been shown to be inversely correlated with ABCB1 expression in the brains of elderly people without dementia, which may indicate that age-related ABCB1 deficiency may be involved in the pathogenesis of Alzheimer’s disease [109][114]. Indeed, normal aging is characterized by a progressive decrease in ABCB1 activity in the blood-brain barrier [115][116]. In this case, ABCB1 is involved in Aβ transport, while disruption of this process may be associated with the development of Alzheimer’s disease [108][109][110][111][112][113].
Thus, ABCB1, in addition to the transport of xenobiotics, is involved in the immune defense of the body, regulating a number of processes in both cells of the innate and adaptive immune systems. These and other data suggest that ABCB1 is a promising target for drug interventions, which are mainly aimed at overcoming multidrug resistance in cancer.
2.3. The ABCC Subfamily
Members of the ABCC subfamily, such as ABCC1 (MRP1), ABCC2 (MRP2), ABCC3 (MRP3), ABCC4 (MRP4), ABCC5 (MRP5), ABCC6 (MRP6), and ABCC10 (MRP7), are known for their roles in multidrug resistance mechanisms [117]. In addition, MRP1 [118], MRP2 [119], MRP3 [120], MRP4 [121], MRP6 [122], MRP7 [123], and MRP8 [124] are involved in LTC 4 transport [125]. MRP1 is also involved in the transport of leukotriene D4 (LTD4) and LTE 4, which are metabolized forms of LTC 4 [118]. MRP1−/− mice have shown an impaired response to inflammatory stimuli, which is associated with decreased LTC 4 secretion [126][127]. ABCC4 also mediates ATP-dependent outflow of LTB 4, which is a hydrolyzed form of the LTA 4 precursor [121][125]. Given that leukotrienes are lipid mediators involved in inflammation, the transport activity of ABCC may be involved in the regulation of inflammation. It has been shown that mrp1−/− mice were resistant to pneumococcal pneumonia, through the increased release of LTB4, which mediates enhanced bacterial clearance in these mice. Increased LTB 4 production is associated with intracellular accumulation of LTC4, a product inhibition of LTC 4 synthase that eliminates substrate competition between LTC4 synthase and LTA4 hydrolase for substrate [128].
ABCC1 is also involved in the export of Sphingosine-1-phosphate (S1P), a lipid mediator involved in many biological processes, including inflammation, angiogenesis, apoptosis, macrophage function, and regulation of endothelial barrier integrity.
In addition, the human multidrug resistance protein MRP4 actively transports the prostaglandins PGE 1 and PGE 2 [127]. Through participation in the transport of PGE 2, MRP4 significantly contributes the migration of human dendritic cells to the draining lymph nodes, which is important for the initiation of the immune response [129][130][131][132]. Inhibition of MRP4 has been shown to reduce the number of migrated skin dendritic cells by 60–70% [132][133][134]. ABCC4 is also involved in the regulation of fibroblast migration. In this process, F-actin was identified as a downstream target and the main mediator of MRP4 effect on cell migration, with cAMP/cGMP playing the role of signaling molecules [134][135].
The role of MRP1 in adaptive immunity is of interest. Adaptive immune responses begin after the transfer of antigen-containing dendritic cells from peripheral tissues to the lymph nodes. In doing so, MRP-1 regulates the migration of dendritic cells into the lymph nodes by transporting LTC4, which promotes chemotaxis to CCL19 and mobilization of dendritic cells from the epidermis [136].
Another member of this subfamily, MRP2 regulates mucosal inflammation by enhancing neutrophil transmigration, which is associated with increased hepoxylin A3 (HXA 3) synthesis [95]. HXA 3 is a biologically relevant eicosanoid formed from the intermediate 12S-hydroperoxy-5Z, 8Z, 10 E, 14 Z-eicosatetraenoic acid (12 S-HpETE) formed in the 12-lipoxygenase pathway of arachidonic acid metabolism through hepoxylin synthase activity [95][137][138][139][140].
2.4. The ABCG Subfamily
Many members of the ABCG subfamily are known for their role in lipid homeostasis, among which ABCG1- and ABCG4-transporters, which are half ABC proteins, are the best characterized. They consist of one transmembrane domain and one nucleotide-binding domain, but for activation, the proteins form a dimer (homodimer or heterodimer) or even an oligomer depending on function [16][141][142][143].
ABCG1 is expressed in many cell types, including myeloid cells, lymphocytes, epithelial and endothelial cells of various organs, and transports cholesterol, 7-ketocholesterol, sphingomyelin, and phosphatidylcholine from cells to HDL [142][144][145]. ABCG1, along with ABCA1, protects cells from sterol overload by removing its export from peripheral cells and saturating them with HDL [143][146][147]. ABCA1 is thought to provide initial cholesterol saturation of lipid-poor apoA-I, thus forming “nascent” HDLs, and then ABCG1 further saturates them with cholesterol [148][149][150][151][152].
ABCG1 is also involved in the regulation of inflammation. Macrophages from Abcg1−/− mice have increased inflammatory activity and had lipid accumulation [153]. Increased levels of the inflammatory cytokines IL-6, IL-1β, IL-1α, and IL-12, and decreased levels of the anti-inflammatory IL-10 in alveolar macrophage cell supernatants were shown. In addition, there was an increase in monocyte recruitment and macrophage differentiation in the lungs of Abcg1−/− mice and increased apoptosis of alveolar macrophages, which generally did not lead to a change in lung macrophage content [153]. At the same time, Abcg1−/− macrophages engulfed many apoptotic cells but could not fully excrete cholesterol derived from these apoptotic cells, which is consistent with the propensity of Abcg1−/− alveolar macrophages for apoptosis [153].
ABCG1 has also been shown to be involved in macrophage polarization. ABCG1 deficiency contributes to proinflammatory M1 polarization of human macrophages. This mechanism is thought to be mediated through the Akt signaling pathway [154]. Another study showed that increased cholesterol accumulation in macrophages in the absence of ABCG1 causes NF-κB activation, which polarizes these macrophages to a proinflammatory M1 phenotype [155]. It has also been shown that ABCG1 deficiency is sufficient to inhibit the chemotaxis of M2 macrophages [156].
Thus, changes in cholesterol homeostasis due to lack of ABCG1 modulate immune cell function. These and other data identified an important role of ABCG1 in the prevention of atherosclerosis, which allows us to consider ABCG1 along with ABCA1 as potential therapeutic targets for the prevention of atherosclerosis through the regulation of their expression, localization, and degradation [157].
This entry is adapted from the peer-reviewed paper 10.3390/membranes12111083
References
- Wang, Z.; Tian, F.; Cai, L.; Zhang, J.; Liu, J.; Zeng, X. Identification of candidate ATP-binding cassette transporter gene family members in Diaphorina citri (Hemiptera: Psyllidae) via adult tissues transcriptome analysis. Sci. Rep. 2019, 9, 15842.
- Dawson, R.J.; Locher, K.P. Structure of a bacterial multidrug ABC transporter. Nature 2006, 443, 180–185.
- Kotlyarov, S.; Kotlyarova, A. The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 6711.
- Jardetzky, O. Simple allosteric model for membrane pumps. Nature 1966, 211, 969–970.
- Eggensperger, S.; Tampé, R. The transporter associated with antigen processing: A key player in adaptive immunity. Biol. Chem. 2015, 396, 1059–1072.
- Phillips, M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018, 59, 749–763.
- Quazi, F.; Molday, R.S. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011, 50, 265–290.
- Francone, O.L.; Royer, L.; Boucher, G.; Haghpassand, M.; Freeman, A.; Brees, D.; Aiello, R.J. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1198–1205.
- Yvan-Charvet, L.; Welch, C.; Pagler, T.A.; Ranalletta, M.; Lamkanfi, M.; Han, S.; Ishibashi, M.; Li, R.; Wang, N.; Tall, A.R. Increased inflammatory gene expression in ABC transporter-deficient macrophages: Free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008, 118, 1837–1847.
- O’Reilly, M.E.; Kajani, S.; Ralston, J.C.; Lenighan, Y.M.; Roche, H.M.; McGillicuddy, F.C. Nutritionally Derived Metabolic Cues Typical of the Obese Microenvironment Increase Cholesterol Efflux Capacity of Adipose Tissue Macrophages. Mol. Nutr. Food Res. 2019, 63, 1800713.
- Lawn, R.M.; Wade, D.P.; Garvin, M.R.; Wang, X.; Schwartz, K.; Porter, J.G.; Seilhamer, J.J.; Vaughan, A.M.; Oram, J.F. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Investig. 1999, 104, R25–R31.
- Wang, N.; Silver, D.L.; Costet, P.; Tall, A.R. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 2000, 275, 33053–33058.
- Neufeld, E.B.; Remaley, A.T.; Demosky, S.J.; Stonik, J.A.; Cooney, A.M.; Comly, M.; Dwyer, N.K.; Zhang, M.; Blanchette-Mackie, J.; Santamarina-Fojo, S.; et al. Cellular localization and trafficking of the human ABCA1 transporter. J. Biol. Chem. 2001, 276, 27584–27590.
- Singaraja, R.R.; Kang, M.H.; Vaid, K.; Sanders, S.S.; Vilas, G.L.; Arstikaitis, P.; Coutinho, J.; Drisdel, R.C.; El-Husseini Ael, D.; Green, W.N.; et al. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ. Res. 2009, 105, 138–147.
- Landry, Y.D.; Denis, M.; Nandi, S.; Bell, S.; Vaughan, A.M.; Zha, X. ATP-binding Cassette Transporter A1 Expression Disrupts Raft Membrane Microdomains through Its ATPase-related Functions. J. Biol. Chem. 2006, 281, 36091–36101.
- Sano, O.; Ito, S.; Kato, R.; Shimizu, Y.; Kobayashi, A.; Kimura, Y.; Kioka, N.; Hanada, K.; Ueda, K.; Matsuo, M. ABCA1, ABCG1, and ABCG4 are distributed to distinct membrane meso-domains and disturb detergent-resistant domains on the plasma membrane. PLoS ONE 2014, 9, e109886.
- Mendez, A.J.; Lin, G.; Wade, D.P.; Lawn, R.M.; Oram, J.F. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 2001, 276, 3158–3166.
- Iatan, I.; Bailey, D.; Ruel, I.; Hafiane, A.; Campbell, S.; Krimbou, L.; Genest, J. Membrane microdomains modulate oligomeric ABCA1 function: Impact on apoAI-mediated lipid removal and phosphatidylcholine biosynthesis. J. Lipid Res. 2011, 52, 2043–2055.
- Yamauchi, Y.; Yokoyama, S.; Chang, T.Y. ABCA1-dependent sterol release: Sterol molecule specificity and potential membrane domain for HDL biogenesis. J. Lipid Res. 2016, 57, 77–88.
- Witting, S.R.; Maiorano, J.N.; Davidson, W.S. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter A1. J. Biol. Chem. 2003, 278, 40121–40127.
- Fantini, J.; Epand, R.M.; Barrantes, F.J. Cholesterol-recognition motifs in membrane proteins. Direct Mech. Cholest. Modul. Protein Funct. 2019, 3–25.
- Ruysschaert, J.-M.; Lonez, C. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta (BBA)-Biomembr. 2015, 1848, 1860–1867.
- Kusnadi, A.; Park, S.H.; Yuan, R.; Pannellini, T.; Giannopoulou, E.; Oliver, D.; Lu, T.; Park-Min, K.H.; Ivashkiv, L.B. The Cytokine TNF Promotes Transcription Factor SREBP Activity and Binding to Inflammatory Genes to Activate Macrophages and Limit Tissue Repair. Immunity 2019, 51, 241–257.e249.
- Sharpe, L.J.; Rao, G.; Jones, P.M.; Glancey, E.; Aleidi, S.M.; George, A.M.; Brown, A.J.; Gelissen, I.C. Cholesterol sensing by the ABCG1 lipid transporter: Requirement of a CRAC motif in the final transmembrane domain. Biochim. Biophys. Acta. 2015, 1851, 956–964.
- Fantini, J.; Barrantes, F.J. How cholesterol interacts with membrane proteins: An exploration of cholesterol-binding sites including CRAC, CARC, and tilted domains. Front. Physiol. 2013, 4, 31.
- Li, H.; Papadopoulos, V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology 1998, 139, 4991–4997.
- Zhu, X.; Owen, J.S.; Wilson, M.D.; Li, H.; Griffiths, G.L.; Thomas, M.J.; Hiltbold, E.M.; Fessler, M.B.; Parks, J.S. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010, 51, 3196–3206.
- Castrillo, A.; Joseph, S.B.; Vaidya, S.A.; Haberland, M.; Fogelman, A.M.; Cheng, G.; Tontonoz, P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell 2003, 12, 805–816.
- Frisdal, E.; Lesnik, P.; Olivier, M.; Robillard, P.; Chapman, M.J.; Huby, T.; Guerin, M.; Le Goff, W. Interleukin-6 protects human macrophages from cellular cholesterol accumulation and attenuates the proinflammatory response. J. Biol. Chem. 2011, 286, 30926–30936.
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912.
- Azzam, K.M.; Fessler, M.B. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol. Metab. 2012, 23, 169–178.
- Thompson, P.A.; Gauthier, K.C.; Varley, A.W.; Kitchens, R.L. ABCA1 promotes the efflux of bacterial LPS from macrophages and accelerates recovery from LPS-induced tolerance. J. Lipid Res. 2010, 51, 2672–2685.
- Burnett, J.R.; Hooper, A.J.; McCormick, S.P.A.; Hegele, R.A. Tangier Disease. In GeneReviews(®); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993.
- Liu, H.-F.; Cui, K.-F.; Wang, J.-P.; Zhang, M.; Guo, Y.-P.; Li, X.-Y.; Jiang, C. Significance of ABCA1 in human carotid atherosclerotic plaques. Exp. Ther. Med. 2012, 4, 297–302.
- Albrecht, C.; Soumian, S.; Amey, J.S.; Sardini, A.; Higgins, C.F.; Davies, A.H.; Gibbs, R.G.J. ABCA1 Expression in Carotid Atherosclerotic Plaques. Stroke 2004, 35, 2801–2806.
- van Eck, M.; Bos, I.S.; Kaminski, W.E.; Orsó, E.; Rothe, G.; Twisk, J.; Böttcher, A.; Van Amersfoort, E.S.; Christiansen-Weber, T.A.; Fung-Leung, W.P.; et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. USA 2002, 99, 6298–6303.
- Thomas, C.; Gautier, T.; Masson, D. Non-lipogenic ABCA1 inducers: The holy grail in cardio-metabolic diseases? eBioMedicine 2021, 66, 103324.
- Aikawa, T.; Ren, Y.; Holm, M.L.; Asmann, Y.W.; Alam, A.; Fitzgerald, M.L.; Bu, G.; Kanekiyo, T. ABCA7 Regulates Brain Fatty Acid Metabolism During LPS-Induced Acute Inflammation. Front. Neurosci. 2021, 15, 647974.
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Yokoyama, S. Roles of ATP-binding cassette transporter A7 in cholesterol homeostasis and host defense system. J. Atheroscler. Thromb. 2011, 18, 274–281.
- Jehle, A.W.; Gardai, S.J.; Li, S.; Linsel-Nitschke, P.; Morimoto, K.; Janssen, W.J.; Vandivier, R.W.; Wang, N.; Greenberg, S.; Dale, B.M.; et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J. Cell Biol. 2006, 174, 547–556.
- Tanaka, N.; Abe-Dohmae, S.; Iwamoto, N.; Fitzgerald, M.L.; Yokoyama, S. Helical apolipoproteins of high-density lipoprotein enhance phagocytosis by stabilizing ATP-binding cassette transporter A7. J. Lipid Res. 2010, 51, 2591–2599.
- Wang, N.; Lan, D.; Gerbod-Giannone, M.; Linsel-Nitschke, P.; Jehle, A.W.; Chen, W.; Martinez, L.O.; Tall, A.R. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J. Biol. Chem. 2003, 278, 42906–42912.
- Ikeda, Y.; Abe-Dohmae, S.; Munehira, Y.; Aoki, R.; Kawamoto, S.; Furuya, A.; Shitara, K.; Amachi, T.; Kioka, N.; Matsuo, M.; et al. Posttranscriptional regulation of human ABCA7 and its function for the apoA-I-dependent lipid release. Biochem. Biophys. Res. Commun. 2003, 311, 313–318.
- Sasaki, M.; Shoji, A.; Kubo, Y.; Nada, S.; Yamaguchi, A. Cloning of rat ABCA7 and its preferential expression in platelets. Biochem. Biophys. Res. Commun. 2003, 304, 777–782.
- Abe-Dohmae, S.; Ikeda, Y.; Matsuo, M.; Hayashi, M.; Okuhira, K.; Ueda, K.; Yokoyama, S. Human ABCA7 supports apolipoprotein-mediated release of cellular cholesterol and phospholipid to generate high density lipoprotein. J. Biol. Chem. 2004, 279, 604–611.
- Iwamoto, N.; Abe-Dohmae, S.; Sato, R.; Yokoyama, S. ABCA7 expression is regulated by cellular cholesterol through the SREBP2 pathway and associated with phagocytosis. J. Lipid Res. 2006, 47, 1915–1927.
- Petry, F.; Ritz, V.; Meineke, C.; Middel, P.; Kietzmann, T.; Schmitz-Salue, C.; Hirsch-Ernst, K.I. Subcellular localization of rat Abca5, a rat ATP-binding-cassette transporter expressed in Leydig cells, and characterization of its splice variant apparently encoding a half-transporter. Biochem. J. 2006, 393, 79–87.
- Schumacher, T.; Benndorf, R.A. ABC Transport Proteins in Cardiovascular Disease-A Brief Summary. Molecules 2017, 22, 589.
- Kotlyarov, S.; Kotlyarova, A. Bioinformatic Analysis of ABCA1 Gene Expression in Smoking and Chronic Obstructive Pulmonary Disease. Membranes 2021, 11, 674.
- Kotlyarov, S.; Bulgakov, A. Lipid Metabolism Disorders in the Comorbid Course of Nonalcoholic Fatty Liver Disease and Chronic Obstructive Pulmonary Disease. Cells 2021, 10, 2978.
- Bossennec, M.; Di Roio, A.; Caux, C.; Ménétrier-Caux, C. MDR1 in immunity: Friend or foe? OncoImmunology 2018, 7, e1499388.
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41.
- Barreto-Ojeda, E.; Corradi, V.; Gu, R.X.; Tieleman, D.P. Coarse-grained molecular dynamics simulations reveal lipid access pathways in P-glycoprotein. J. Gen. Physiol. 2018, 150, 417–429.
- Sharom, F.J. The P-glycoprotein efflux pump: How does it transport drugs? J. Membr. Biol. 1997, 160, 161–175.
- Subramanian, N.; Condic-Jurkic, K.; O’Mara, M.L. Structural and dynamic perspectives on the promiscuous transport activity of P-glycoprotein. Neurochem. Int. 2016, 98, 146–152.
- Siarheyeva, A.; Lopez, J.J.; Glaubitz, C. Localization of Multidrug Transporter Substrates within Model Membranes. Biochemistry 2006, 45, 6203–6211.
- Higgins, C.F.; Gottesman, M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992, 17, 18–21.
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559.
- Seelig, A. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 2007, 33, 32–41.
- Gerebtzoff, G.; Seelig, A. In silico prediction of blood-brain barrier permeation using the calculated molecular cross-sectional area as main parameter. J. Chem. Inf. Model. 2006, 46, 2638–2650.
- Neumann, J.; Rose-Sperling, D.; Hellmich, U.A. Diverse relations between ABC transporters and lipids: An overview. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 605–618.
- Batetta, B.; Dessì, S.; Putzolu, M.; Sanna, F.; Spano, O.; Mulas, M.F.; Petruzzo, P.; Cappai, A.; Brotzu, G. MDR1 gene expression in normal and atherosclerotic human arteries(1). J. Vasc. Res. 1999, 36, 261–271.
- van Helvoort, A.; Smith, A.J.; Sprong, H.; Fritzsche, I.; Schinkel, A.H.; Borst, P.; van Meer, G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell 1996, 87, 507–517.
- Kimura, Y.; Kioka, N.; Kato, H.; Matsuo, M.; Ueda, K. Modulation of drug-stimulated ATPase activity of human MDR1/P-glycoprotein by cholesterol. Biochem. J. 2007, 401, 597–605.
- Garrigues, A.; Escargueil, A.E.; Orlowski, S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc. Natl. Acad. Sci. USA 2002, 99, 10347–10352.
- Clay, A.T.; Lu, P.; Sharom, F.J. Interaction of the P-glycoprotein multidrug transporter with sterols. Biochemistry 2015, 54, 6586–6597.
- Bosch, I.; Dunussi-Joannopoulos, K.; Wu, R.-L.; Furlong, S.T.; Croop, J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry 1997, 36, 5685–5694.
- Grybauskas, A.; Koga, T.; Kuprys, P.V.; Nolan, M.; McCarty, R.; Walker, L.; Green, K.A.; Norkett, W.M.; Yue, B.Y.J.T.; Knepper, P.A. ABCB1 transporter and Toll-like receptor 4 in trabecular meshwork cells. Mol. Vis. 2015, 21, 201–212.
- Barancík, M.; Bohácová, V.; Kvackajová, J.; Hudecová, S.; Krizanová, O.; Breier, A. SB203580, a specific inhibitor of p38-MAPK pathway, is a new reversal agent of P-glycoprotein-mediated multidrug resistance. Eur. J. Pharm. Sci. 2001, 14, 29–36.
- Matsuzawa, A.; Saegusa, K.; Noguchi, T.; Sadamitsu, C.; Nishitoh, H.; Nagai, S.; Koyasu, S.; Matsumoto, K.; Takeda, K.; Ichijo, H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 2005, 6, 587–592.
- Frank, M.; Hennenberg, E.M.; Eyking, A.; Rünzi, M.; Gerken, G.; Scott, P.; Parkhill, J.; Walker, A.W.; Cario, E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J. Immunol. 2015, 194, 1983–1995.
- Mottaz, H.; Schönenberger, R.; Fischer, S.; Eggen, R.I.L.; Schirmer, K.; Groh, K.J. Dose-dependent effects of morphine on lipopolysaccharide (LPS)-induced inflammation, and involvement of multixenobiotic resistance (MXR) transporters in LPS efflux in teleost fish. Environ. Pollut. 2017, 221, 105–115.
- Kooij, G.; Backer, R.; Koning, J.J.; Reijerkerk, A.; van Horssen, J.; van der Pol, S.M.; Drexhage, J.; Schinkel, A.; Dijkstra, C.D.; den Haan, J.M.; et al. P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS ONE 2009, 4, e8212.
- Sigal, N.; Kaplan Zeevi, M.; Weinstein, S.; Peer, D.; Herskovits, A.A. The human P-glycoprotein transporter enhances the type I interferon response to Listeria monocytogenes infection. Infect. Immun. 2015, 83, 2358–2368.
- Wyska, E. Pretreatment with R(+)-verapamil significantly reduces mortality and cytokine expression in murine model of septic shock. Int. Immunopharmacol. 2009, 9, 478–490.
- Pendse, S.S.; Behjati, S.; Schatton, T.; Izawa, A.; Sayegh, M.H.; Frank, M.H. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am. J. Transpl. 2006, 6, 2884–2893.
- Frank, M.H.; Denton, M.D.; Alexander, S.I.; Khoury, S.J.; Sayegh, M.H.; Briscoe, D.M. Specific MDR1 P-glycoprotein blockade inhibits human alloimmune T cell activation in vitro. J. Immunol. 2001, 166, 2451–2459.
- Lloberas, N.; Rama, I.; Llaudó, I.; Torras, J.; Cerezo, G.; Cassis, L.; Franquesa, M.; Merino, A.; Benitez-Ribas, D.; Cruzado, J.M.; et al. Dendritic cells phenotype fitting under hypoxia or lipopolysaccharide; adenosine 5’-triphosphate-binding cassette transporters far beyond an efflux pump. Clin. Exp. Immunol. 2013, 172, 444–454.
- Randolph, G.J.; Beaulieu, S.; Pope, M.; Sugawara, I.; Hoffman, L.; Steinman, R.M.; Muller, W.A. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc. Natl. Acad. Sci. USA 1998, 95, 6924–6929.
- Raggers, R.J.; Vogels, I.; van Meer, G. Multidrug-resistance P-glycoprotein (MDR1) secretes platelet-activating factor. Biochem. J. 2001, 357, 859–865.
- Mizutani, T.; Masuda, M.; Nakai, E.; Furumiya, K.; Togawa, H.; Nakamura, Y.; Kawai, Y.; Nakahira, K.; Shinkai, S.; Takahashi, K. Genuine functions of P-glycoprotein (ABCB1). Curr. Drug Metab. 2008, 9, 167–174.
- Panwala, C.M.; Jones, J.C.; Viney, J.L. A novel model of inflammatory bowel disease: Mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J. Immunol. 1998, 161, 5733–5744.
- Foley, S.E.; Tuohy, C.; Dunford, M.; Grey, M.J.; De Luca, H.; Cawley, C.; Szabady, R.L.; Maldonado-Contreras, A.; Houghton, J.M.; Ward, D.V.; et al. Gut microbiota regulation of P-glycoprotein in the intestinal epithelium in maintenance of homeostasis. Microbiome 2021, 9, 183.
- Foley, S.E.; Dente, M.J.; Lei, X.; Sallis, B.F.; Loew, E.B.; Meza-Segura, M.; Fitzgerald, K.A.; McCormick, B.A. Microbial Metabolites Orchestrate a Distinct Multi-Tiered Regulatory Network in the Intestinal Epithelium That Directs P-Glycoprotein Expression. mBio 2022, 13, e0199322.
- Mickley, L.A.; Bates, S.E.; Richert, N.D.; Currier, S.; Tanaka, S.; Foss, F.; Rosen, N.; Fojo, A.T. Modulation of the expression of a multidrug resistance gene (mdr-1/P-glycoprotein) by differentiating agents. J. Biol. Chem. 1989, 264, 18031–18040.
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283.
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662.
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670.e655.
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Investig. 2018, 128, 4044–4056.
- Andersen, V.; Svenningsen, K.; Knudsen, L.A.; Hansen, A.K.; Holmskov, U.; Stensballe, A.; Vogel, U. Novel understanding of ABC transporters ABCB1/MDR/P-glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J. Gastroenterol. 2015, 21, 11862–11876.
- Paik, J.; Fierce, Y.; Treuting, P.M.; Brabb, T.; Maggio-Price, L. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a−/− male mice. J. Nutr. 2013, 143, 1240–1247.
- Englund, G.; Jacobson, A.; Rorsman, F.; Artursson, P.; Kindmark, A.; Rönnblom, A. Efflux transporters in ulcerative colitis: Decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm. Bowel Dis. 2007, 13, 291–297.
- Langmann, T.; Moehle, C.; Mauerer, R.; Scharl, M.; Liebisch, G.; Zahn, A.; Stremmel, W.; Schmitz, G. Loss of detoxification in inflammatory bowel disease: Dysregulation of pregnane X receptor target genes. Gastroenterology 2004, 127, 26–40.
- Andersen, V.; Vogel, U.; Godiksen, S.; Frenzel, F.B.; Sæbø, M.; Hamfjord, J.; Kure, E.; Vogel, L.K. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS ONE 2013, 8, e72119.
- Pazos, M.; Siccardi, D.; Mumy, K.L.; Bien, J.D.; Louie, S.; Shi, H.N.; Gronert, K.; Mrsny, R.J.; McCormick, B.A. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J. Immunol. 2008, 181, 8044–8052.
- Neudeck, B.L.; Loeb, J.M.; Faith, N.G.; Czuprynski, C.J. Intestinal P glycoprotein acts as a natural defense mechanism against Listeria monocytogenes. Infect. Immun. 2004, 72, 3849–3854.
- Mercado-Lubo, R.; McCormick, B.A. The interaction of gut microbes with host ABC transporters. Gut Microbes 2010, 1, 301–306.
- Siccardi, D.; Mumy, K.L.; Wall, D.M.; Bien, J.D.; McCormick, B.A. Salmonella enterica serovar Typhimurium modulates P-glycoprotein in the intestinal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1392–G1400.
- Mercado-Lubo, R.; Zhang, Y.; Zhao, L.; Rossi, K.; Wu, X.; Zou, Y.; Castillo, A.; Leonard, J.; Bortell, R.; Greiner, D.L.; et al. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat. Commun. 2016, 7, 12225.
- Becerra-Báez, E.I.; Meza-Toledo, S.E.; Muñoz-López, P.; Flores-Martínez, L.F.; Fraga-Pérez, K.; Magaño-Bocanegra, K.J.; Juárez-Hernández, U.; Mateos-Chávez, A.A.; Luria-Pérez, R. Recombinant Attenuated Salmonella enterica as a Delivery System of Heterologous Molecules in Cancer Therapy. Cancers 2022, 14, 4224.
- Ye, S.; MacEachran, D.P.; Hamilton, J.W.; O’Toole, G.A.; Stanton, B.A. Chemotoxicity of doxorubicin and surface expression of P-glycoprotein (MDR1) is regulated by the Pseudomonas aeruginosa toxin Cif. Am. J. Physiol. Cell Physiol. 2008, 295, C807–C818.
- Wirths, S.; Lanzavecchia, A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur. J. Immunol. 2005, 35, 3433–3441.
- Pierce, S.K. Lipid rafts and B-cell activation. Nat. Rev. Immunol. 2002, 2, 96–105.
- Weng, H.J.; Tsai, T.F. ABCB1 in dermatology: Roles in skin diseases and their treatment. J. Mol. Med. 2021, 99, 1527–1538.
- Hashimoto, N.; Nakamichi, N.; Nanmo, H.; Kimura, K.-i.; Masuo, Y.; Sakai, Y.; Schinkel, A.H.; Sato, S.; Soga, T.; Kato, Y. Metabolome Analysis Reveals Dermal Histamine Accumulation in Murine Dermatitis Provoked by Genetic Deletion of P-Glycoprotein and Breast Cancer Resistance Protein. Pharm. Res. 2019, 36, 158.
- Mizuno, K.; Akimoto, N.; Kawamura, M.; Nakase, K.; Noguchi, N.; Sato, T. Involvement of adenosine triphosphate-binding cassette subfamily B member 1 in the augmentation of triacylglycerol excretion by Propionibacterium acnes in differentiated hamster sebocytes. J. Dermatol. 2017, 44, 1404–1407.
- Landreville, S.; Agapova, O.A.; Kneass, Z.T.; Salesse, C.; Harbour, J.W. ABCB1 identifies a subpopulation of uveal melanoma cells with high metastatic propensity. Pigment Cell Melanoma Res. 2011, 24, 430–437.
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.M.; Sharom, F.J.; Reiner, P.B. beta-Amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128.
- Vogelgesang, S.; Cascorbi, I.; Schroeder, E.; Pahnke, J.; Kroemer, H.K.; Siegmund, W.; Kunert-Keil, C.; Walker, L.C.; Warzok, R.W. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics 2002, 12, 535–541.
- Vogelgesang, S.; Warzok, R.W.; Cascorbi, I.; Kunert-Keil, C.; Schroeder, E.; Kroemer, H.K.; Siegmund, W.; Walker, L.C.; Pahnke, J. The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 121–125.
- Cirrito, J.R.; Deane, R.; Fagan, A.M.; Spinner, M.L.; Parsadanian, M.; Finn, M.B.; Jiang, H.; Prior, J.L.; Sagare, A.; Bales, K.R.; et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Investig. 2005, 115, 3285–3290.
- Kuhnke, D.; Jedlitschky, G.; Grube, M.; Krohn, M.; Jucker, M.; Mosyagin, I.; Cascorbi, I.; Walker, L.C.; Kroemer, H.K.; Warzok, R.W.; et al. MDR1-P-Glycoprotein (ABCB1) Mediates Transport of Alzheimer’s amyloid-beta peptides—Implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007, 17, 347–353.
- Silverberg, G.D.; Messier, A.A.; Miller, M.C.; Machan, J.T.; Majmudar, S.S.; Stopa, E.G.; Donahue, J.E.; Johanson, C.E. Amyloid efflux transporter expression at the blood-brain barrier declines in normal aging. J. Neuropathol. Exp. Neurol. 2010, 69, 1034–1043.
- Vogelgesang, S.; Glatzel, M.; Walker, L.C.; Kroemer, H.K.; Aguzzi, A.; Warzok, R.W. Cerebrovascular P-glycoprotein expression is decreased in Creutzfeldt-Jakob disease. Acta Neuropathol. 2006, 111, 436–443.
- Bartels, A.L. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr. Pharm. Des. 2011, 17, 2771–2777.
- van Assema, D.M.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood-brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776.
- Tian, Q.; Zhang, J.; Chan, E.; Duan, W.; Zhou, S. Multidrug resistance proteins (MRPs) and implication in drug development. Drug Dev. Res. 2005, 64, 1–18.
- Leier, I.; Jedlitschky, G.; Buchholz, U.; Cole, S.P.; Deeley, R.G.; Keppler, D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J. Biol. Chem. 1994, 269, 27807–27810.
- Cui, Y.; König, J.; Buchholz, U.; Spring, H.; Leier, I.; Keppler, D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 1999, 55, 929–937.
- Zeng, H.; Liu, G.; Rea, P.A.; Kruh, G.D. Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res. 2000, 60, 4779–4784.
- Rius, M.; Hummel-Eisenbeiss, J.; Keppler, D. ATP-dependent transport of leukotrienes B4 and C4 by the multidrug resistance protein ABCC4 (MRP4). J. Pharm. Exp. 2008, 324, 86–94.
- Belinsky, M.G.; Chen, Z.S.; Shchaveleva, I.; Zeng, H.; Kruh, G.D. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 2002, 62, 6172–6177.
- Chen, Z.S.; Hopper-Borge, E.; Belinsky, M.G.; Shchaveleva, I.; Kotova, E.; Kruh, G.D. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10). Mol. Pharm. 2003, 63, 351–358.
- Chen, Z.S.; Guo, Y.; Belinsky, M.G.; Kotova, E.; Kruh, G.D. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol. Pharm. 2005, 67, 545–557.
- van de Ven, R.; Oerlemans, R.; van der Heijden, J.W.; Scheffer, G.L.; de Gruijl, T.D.; Jansen, G.; Scheper, R.J. ABC drug transporters and immunity: Novel therapeutic targets in autoimmunity and cancer. J. Leukoc. Biol. 2009, 86, 1075–1087.
- Wijnholds, J.; Evers, R.; van Leusden, M.R.; Mol, C.A.; Zaman, G.J.; Mayer, U.; Beijnen, J.H.; van der Valk, M.; Krimpenfort, P.; Borst, P. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat. Med. 1997, 3, 1275–1279.
- Reid, G.; Wielinga, P.; Zelcer, N.; van der Heijden, I.; Kuil, A.; de Haas, M.; Wijnholds, J.; Borst, P. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2003, 100, 9244–9249.
- Schultz, M.J.; Wijnholds, J.; Peppelenbosch, M.P.; Vervoordeldonk, M.J.; Speelman, P.; van Deventer, S.J.; Borst, P.; van der Poll, T. Mice lacking the multidrug resistance protein 1 are resistant to Streptococcus pneumoniae-induced pneumonia. J. Immunol. 2001, 166, 4059–4064.
- Luft, T.; Jefford, M.; Luetjens, P.; Toy, T.; Hochrein, H.; Masterman, K.A.; Maliszewski, C.; Shortman, K.; Cebon, J.; Maraskovsky, E. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: Prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 2002, 100, 1362–1372.
- Scandella, E.; Men, Y.; Legler, D.F.; Gillessen, S.; Prikler, L.; Ludewig, B.; Groettrup, M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood 2004, 103, 1595–1601.
- Legler, D.F.; Krause, P.; Scandella, E.; Singer, E.; Groettrup, M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006, 176, 966–973.
- van de Ven, R.; Scheffer, G.L.; Reurs, A.W.; Lindenberg, J.J.; Oerlemans, R.; Jansen, G.; Gillet, J.-P.; Glasgow, J.N.; Pereboev, A.; Curiel, D.T.; et al. A role for multidrug resistance protein 4 (MRP4; ABCC4) in human dendritic cell migration. Blood 2008, 112, 2353–2359.
- van de Ven, R.; de Groot, J.; Reurs, A.W.; Wijnands, P.G.; van de Wetering, K.; Schuetz, J.D.; de Gruijl, T.D.; Scheper, R.J.; Scheffer, G.L. Unimpaired immune functions in the absence of Mrp4 (Abcc4). Immunol. Lett. 2009, 124, 81–87.
- Kryczka, J.; Boncela, J. Cell Migration Related to MDR-Another Impediment to Effective Chemotherapy? Molecules 2018, 23, 331.
- Sinha, C.; Arora, K.; Naren, A.P. Methods to Study Mrp4-containing Macromolecular Complexes in the Regulation of Fibroblast Migration. J. Vis. Exp. 2016, 53973.
- Robbiani, D.F.; Finch, R.A.; Jäger, D.; Muller, W.A.; Sartorelli, A.C.; Randolph, G.J. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 2000, 103, 757–768.
- Pace-Asciak, C.R.; Reynaud, D.; Demin, P.; Nigam, S. The hepoxilins. A review. Adv. Exp. Med. Biol. 1999, 447, 123–132.
- Nigam, S.; Zafiriou, M.P.; Deva, R.; Ciccoli, R.; Roux-Van der Merwe, R. Structure, biochemistry and biology of hepoxilins: An update. FEBS J. 2007, 274, 3503–3512.
- Nigam, S.; Patabhiraman, S.; Ciccoli, R.; Ishdorj, G.; Schwarz, K.; Petrucev, B.; Kühn, H.; Haeggström, J.Z. The rat leukocyte-type 12-lipoxygenase exhibits an intrinsic hepoxilin A3 synthase activity. J. Biol. Chem. 2004, 279, 29023–29030.
- Yu, Z.; Schneider, C.; Boeglin, W.E.; Marnett, L.J.; Brash, A.R. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. USA 2003, 100, 9162–9167.
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802.
- Cserepes, J.; Szentpétery, Z.; Seres, L.; Özvegy-Laczka, C.; Langmann, T.; Schmitz, G.; Glavinas, H.; Klein, I.; Homolya, L.; Váradi, A. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: Indications for heterodimerization. Biochem. Biophys. Res. Commun. 2004, 320, 860–867.
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779.
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098.
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldán, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005, 1, 121–131.
- Bensinger, S.J.; Bradley, M.N.; Joseph, S.B.; Zelcer, N.; Janssen, E.M.; Hausner, M.A.; Shih, R.; Parks, J.S.; Edwards, P.A.; Jamieson, B.D. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 2008, 134, 97–111.
- Out, R.; Hoekstra, M.; Habets, K.; Meurs, I.; de Waard, V.; Hildebrand, R.B.; Wang, Y.; Chimini, G.; Kuiper, J.; Van Berkel, T.J. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 258–264.
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443.
- Gelissen, I.C.; Harris, M.; Rye, K.A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 534–540.
- Jessup, W.; Gelissen, I.C.; Gaus, K.; Kritharides, L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 2006, 17, 247–257.
- Oram, J.F.; Vaughan, A.M. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006, 99, 1031–1043.
- Seres, L.; Cserepes, J.; Elkind, N.B.; Törocsik, D.; Nagy, L.; Sarkadi, B.; Homolya, L. Functional ABCG1 expression induces apoptosis in macrophages and other cell types. Biochim. Biophys. Acta 2008, 1778, 2378–2387.
- Wojcik, A.J.; Skaflen, M.D.; Srinivasan, S.; Hedrick, C.C. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J. Immunol. 2008, 180, 4273–4282.
- Sag, D.; Purcu, D.U.; Altunay, M. The cholesterol transporter ABCG1 modulates macrophage polarization in human monocyte-derived macrophages. J. Immunol. 2019, 202, 187-22.
- Sag, D.; Cekic, C.; Wu, R.; Linden, J.; Hedrick, C.C. The cholesterol transporter ABCG1 links cholesterol homeostasis and tumour immunity. Nat. Commun. 2015, 6, 6354.
- Wei, H.; Tarling, E.J.; McMillen, T.S.; Tang, C.; LeBoeuf, R.C. ABCG1 regulates mouse adipose tissue macrophage cholesterol levels and ratio of M1 to M2 cells in obesity and caloric restriction. J. Lipid Res. 2015, 56, 2337–2347.
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203.
This entry is offline, you can click here to edit this entry!
