Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Functional polymeric biomaterials (FPBMs) represent the most popular usage of natural, synthetic, or hybrid polymeric materials interacting with biological regimes and are used to protect against microbes and regenerate, repair, and treat any type of tissue in the organs, or improve the functions of the human body. FPBMs with bioactive characteristics obtained by radiation-induced graft copolymerisation (RIGC) have been subjected to intensive research and developed into many commercial products. Various studies have reported the development of a variety of radiation-grafted FPBMs.
- functional polymeric biomaterials
- radiation induced grafting
- medical and biomedical applications
1. Introduction
Functional polymeric biomaterials (FPBMs) are a class of materials that is receiving increasing interest because of their relevance to applications in various areas impacting human life and living [1]. FPBMs represent the most popular usage of natural, synthetic, or hybrid polymeric materials interacting with biological regimes and are used to protect against microbes and regenerate, repair, and treat any type of tissue in the organs, or improve the functions of the human body [2]. Thus, the research on FPBMs has enabled the development of various implants, medical devices, drug carriers, and scaffolds for tissue engineering with greater antimicrobial resistance, biocompatibility, and biofunctionality together with lower cytotoxicity against malignant cells [3]. FPBMs can be developed using various methods involving surface treatment such as etching, metallisation, ion sputtering, chemical grafting, and radiation-induced graft copolymerisation (RIGC) with low-energy radiation sources (plasma, laser treatment, and UV lamp) and high-energy radiation sources such as γ-rays and electron beam (EB) [4]. Each modification method has its pros and cons, and the selection of a particular method usually controls the developed topical structure and the level as well as the stability of the enhanced properties conferred to the polymer substrates.
Of all methods, RIGC is a distinctive technique for modification of polymer substrates that has been known for the past six decades. The versatility of this method is derived from its ability to permanently modify polymeric substrates by imparting new functionalities originated for the incoming polar monomers (acrylic or vinylic monomers) without compromising the inherent properties of the parent polymers. Such versatility enabled this technology to be used to develop many hybrid polymeric materials with desired properties [5][6][7][8]. The advantages of this method over its well-established chemically induced counterpart are not only in its ability to meticulously control the grafting yield by manipulation of the grafting parameters and the absence of detrimental impurities that maintain the purity of the product but also in the consumption of fewer chemicals and the ability to overcome product shaping problems. This is because grafting reactions can be started with polymer substrates of different physical forms, including films, non-woven fabric, particles, and fibres. Moreover, RIGC is also capable of achieving bulk modifications in the substrates when using γ-rays and EB sources, with the capability to scale up grafted products compared to plasma- and UV-induced grafting, which are limited to laboratory scale [9][10].
RIGC works by exposing a polymer substrate to ionising radiation, leading to the generation of active sites or radicals. The formed radicals initiate copolymerisation when exposed to monomer molecules, forming macroradicals that allow propagation of the molecular chains to form side chain grafts when terminated. RIGC can be carried out by the direct interaction between the monomer and the polymer substrate during irradiation, and this method is called simultaneous radiation grafting. Alternatively, the polymer substrate is initially irradiated and subsequently brought in contact with the monomer molecules in a separate step, and the technique is called pre-irradiation grafting. Introducing functional groups can be made by direct grafting of functional (acrylic) monomers or by a post-grafting reaction upon grafting vinylic monomers. Figure 1 shows a schematic diagram representing the preparation of functional polymeric materials by irradiation, grafting of the monomer, and subsequent functionalisation. More details on RIGC methods, including their advantages and disadvantages, can be found elsewhere [11].
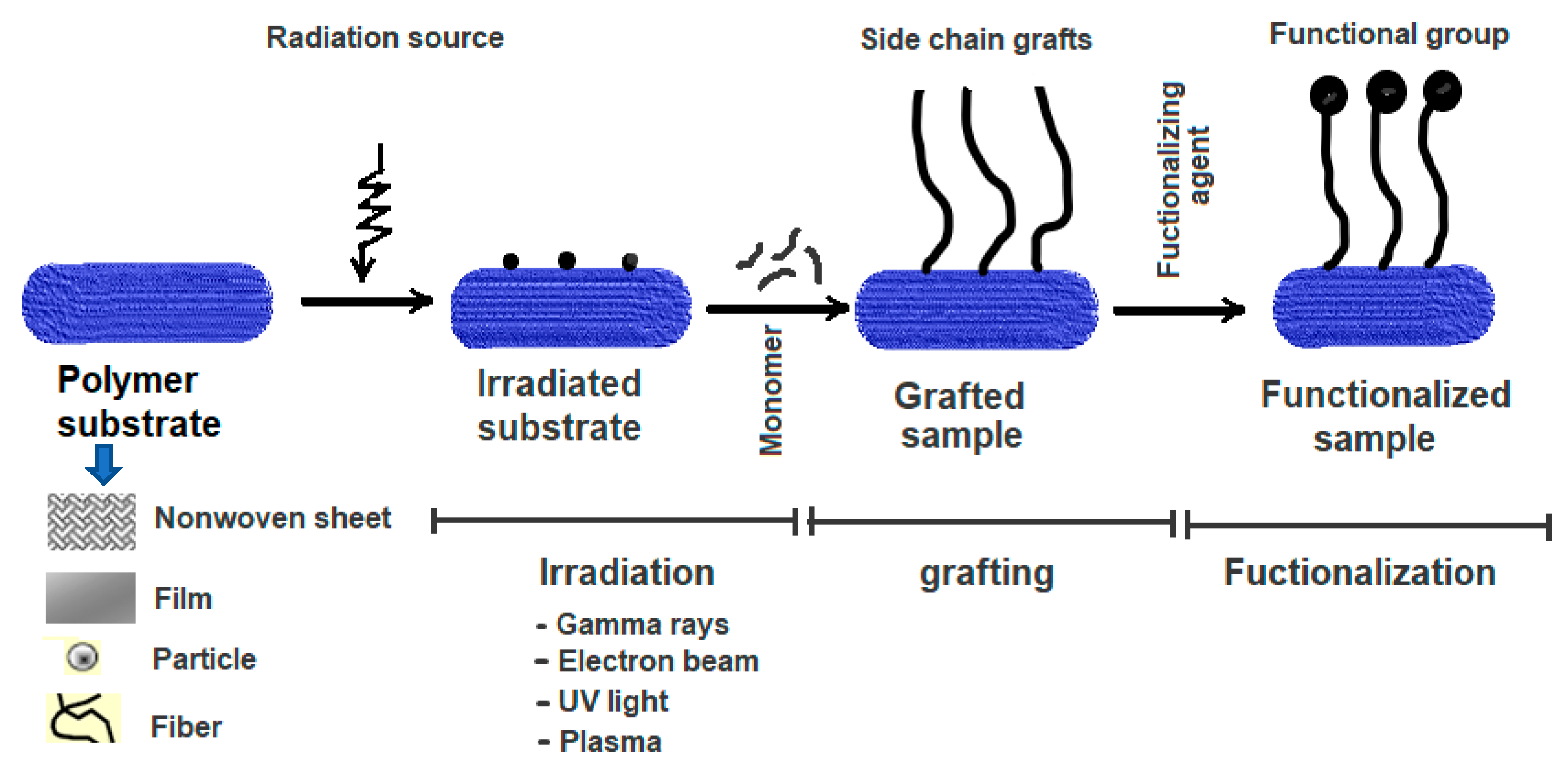
Figure 1. Schematic representation for preparation of functional polymeric materials by RIGC and functionalisation.
RIGC has been extensively used to endow interesting functional characteristics such as hydrophilicity, hydrophobicity, ion conduction, metal ion binding capacity, adhesion, microbial and fouling resistance, and biocompatibility to different polymer substrates. This led to the development of various functional polymeric materials through the integration of various monomer/polymer combinations enduring desired characteristics to polymer substrates. The resultant radiation-grafted functional materials’ applications include battery separators [12][13][14], polymer electrolyte membranes [15][16], chelating adsorbents [17][18][19], ion exchange membranes [20][21], and FPBMs [6][22].
Particularly, the application of RIGC for the development of FPBMs is one of the areas that has received paramount efforts since the early days of the technique’s invention by A. Chapiro (a French scientist) [23] and A. Charlesby (a British scientist) [24]. Subsequently, many researchers used this technique to make enormous contributions to designing and developing innovative materials for medical, biomedical, health care, and biotechnological applications. Figure 2 shows some examples of FPBMs prepared by RIGC for various applications. This leads to the emergence of many potential radiation-grafted materials with great potential for commercialisation, and this field seems to be broadly opening for imaginative future developments.
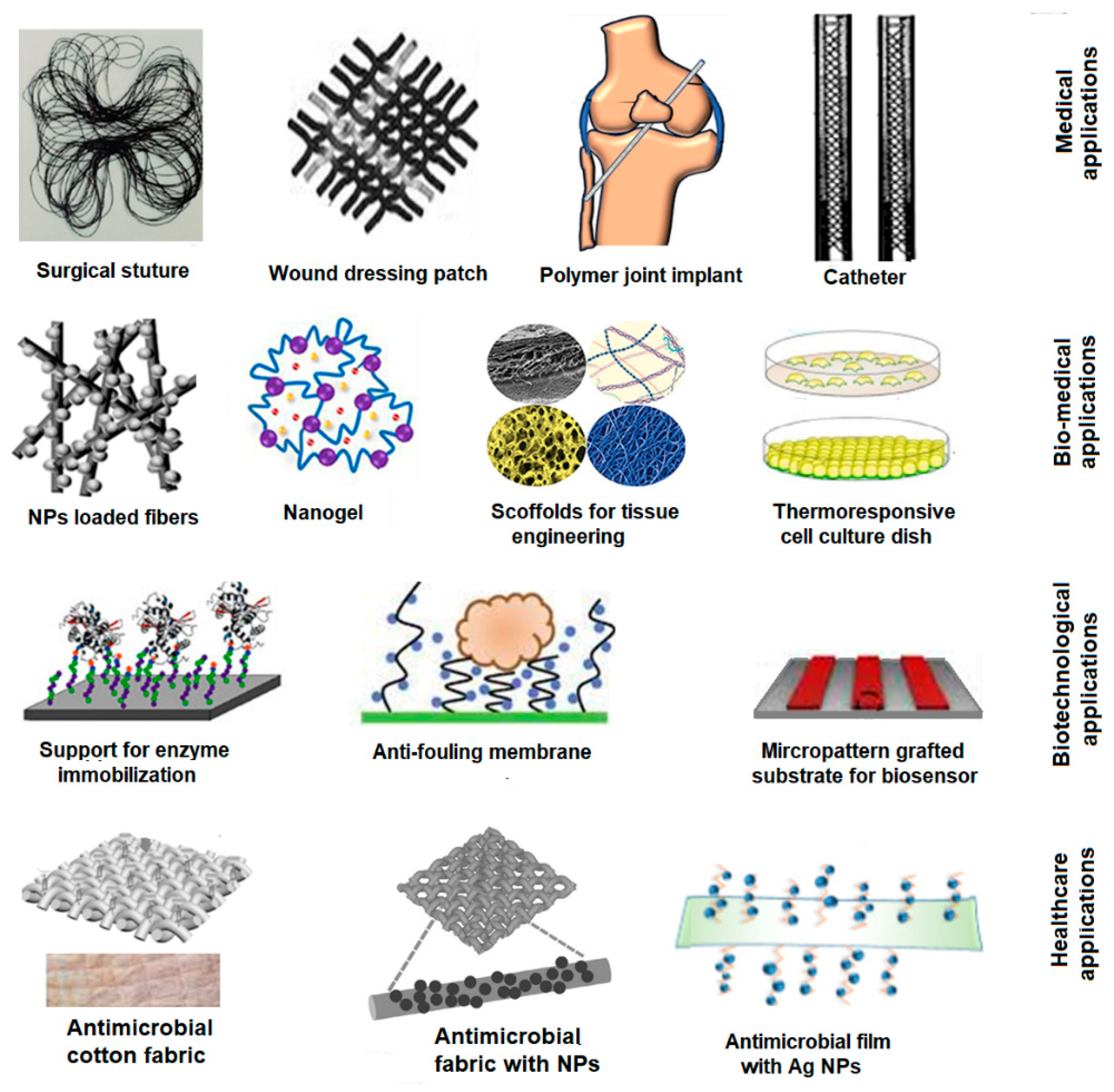
Figure 2. Schematic diagram of examples of FPBMs prepared by RIGC for various applications.
Several articles have been devoted to reviewing the use of RIGC for the development of adsorbents for purifications and separations in environmental applications, including wastewater treatment and CO2 capture [25][26], polymer electrolyte membranes and separators for energy conversion and storage [9][10][27], and protein capture [21]. The preparation and applications of biomaterials such as stimuli-responsive polymer systems and their polymer-biomolecule (protein) conjugates were also reviewed on two occasions [28][29]. Likewise, the use of RIGC or crosslinking to produce hydrogels of various size ranges (microgels and nanogels) and their applications in various medical and biomedical applications were extensively reviewed in many publications [30][31][32][33][34] with some attention recently paid to reviewing current approaches for crosslinking polysaccharides for hydrogel formation [35]. On the other hand, FPBMs obtained by RIGC have also been reviewed on various occasions, but with the focus limited to biomedical applications [6][26][27][28][29], cell sheet engineering, and the characteristics of thermo-responsive scaffolds [36][37]. Most of the published reviews mainly discussed strategies to construct a variety of radiation-grafted biomaterials with desired structure, properties, dynamic functionality, and biological complexity, taking the target application into account and elaborating on the challenges to the endurance of the imparted functionality.
2. Classification and Applications of FPBMs Prepared by RIGC
The design of FPBMs by RIGC was found to focus on the broad aspects of polymer surface modifications, inculcating antimicrobial resistance, biofilm formation prevention, biocompatibility, cytotoxicity resistance, bio-functionality, control-release of therapeutic agents in drug delivery, and healing functionalities in tissue engineering and regenerative medicine [2]. The advantages of this method for the preparation of FPBMs include the use of high-energy radiation for clean initiation (without chemical initiators) and the ability to obtain sterilised products [38]. Applications of FPBMs prepared by RIGC have been frequently reported in the literature, which prevailed the emergence of many urgently needed innovative materials capable of meeting the requirements of diversified applications [6]. Particularly, radiation-grafted FPBMs, such as drug release carriers [39][40], antimicrobial surgical sutures [41], thermo-responsive cell culture plates [42], scaffolds for tissue engineering [43], water-absorbing polymers [44], enzyme carriers [45], antimicrobial catheters [46], implants [47], antimicrobial gauze [48], antimicrobial fabrics [49][50], antibacterial food packing films [51][52], and antifouling membranes [53], have been widely investigated to design efficient, safe, and viable products. Such a wide spectrum of applications emphasises a need for a deep understanding of the knowledge underlying not only radiation chemistry and radiation processing of polymers but also biomedicine, biochemistry, and biomaterials characteristics. The various applications of FPBMs can be classified into four categories, including: (i) medicine, (ii) biomedicine, (iii) biotechnology, and (iv) healthcare, as shown in Table 1.
Table 1. Classification of FPBMs prepared by RIGC.
| Field | Applications | References |
|---|---|---|
| Medical | Implants | [47][54][55][56][57][58][59][60][61] |
| Catheter | [45][46][62][63][64][65][66][67][68] | |
| Surgical sutures | [41][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85] | |
| Wound dressing hydrogels/patches | [48][86][87][88][89][90][91][92] | |
| Biomedical | Scaffolds for tissue engineering | [43][93][94][95][96][97][98][99][100][101][102][103][104] |
| Cell culture plates | [42][105][106][107][108][109][110][111][112][113][114] | |
| Drug release carrier/delivery | [39][40][41][70][89][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141] | |
| Biotechnological | Biosensors | [116][135][136][137][138][139][140] |
| Support for enzyme immobilization/release | [45][142][143][144][145][146][147][148][149][150] | |
| Antifouling membranes | [53][151][152][153][154][155][156][157][158][159][160][161] | |
| Health care | Antimicrobial fabrics and films | [50][162][163][164][165][166][167][168][169][170][171][172][173][174][175][176][177][178] |
| Protective face mask | [49][50][179] | |
| Packaging films | [51][52][180][181] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14224831
References
- Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105.
- Liang, Y.; Li, L.; Scott, R.A.; Kiick, K.L. 50th anniversary perspective: Polymeric biomaterials: Diverse functions enabled by advances in macromolecular chemistry. Macromolecules 2017, 50, 483–502.
- Osorio-Delgado, M.A.; Henao-Tamayo, L.J.; Velásquez-Cock, J.A.; Cañas-Gutierrez, A.I.; Restrepo-Múnera, L.M.; Gañán-Rojo, P.F.; Zuluaga-Gallego, R.O.; Ortiz-Trujillo, I.C.; Castro-Herazo, C.I. Biomedical applications of polymeric biomaterials. Dyna 2017, 84, 241–252.
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115.
- Nasef, M.M.; Hegazy, E.S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561.
- Pino-Ramos, V.H.; Ramos-Ballesteros, A.; López-Saucedo, F.; López-Barriguete, J.E.; Varca, G.H.; Bucio, E. Radiation grafting for the functionalization and development of smart polymeric materials. Top. Curr. Chem. 2016, 374, 63.
- Small, M.; Faglie, A.; Craig, A.J.; Pieper, M.; Fernand Narcisse, V.E.; Neuenschwander, P.F.; Chou, S.-F. Nanostructure-enabled and macromolecule-grafted surfaces for biomedical applications. Micromachines 2018, 9, 243.
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877.
- Nasef, M.M. Radiation-grafted membranes for polymer electrolyte fuel cells: Current trends and future directions. Chem. Rev. 2014, 114, 12278–12329.
- Nasef, M.M.; Gürsel, S.A.; Karabelli, D.; Güven, O. Radiation-grafted materials for energy conversion and energy storage applications. Prog. Polym. Sci. 2016, 63, 1–41.
- Nasef, M.M.; Gupta, B.; Shameli, K.; Verma, C.; Ali, R.R.; Ting, T.M. Engineered Bioactive Polymeric Surfaces by Radiation Induced Graft Copolymerization: Strategies and Applications. Polymers 2021, 13, 3102.
- Ishigaki, I.; Sugo, T.; Senoo, K.; Okada, T.; Okamoto, J.; Machi, S. Graft polymerization of acrylic acid onto polyethylene film by preirradiation method. I. Effects of preirradiation dose, monomer concentration, reaction temperature, and film thickness. J. Appl. Polym. Sci. 1982, 27, 1033–1041.
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012.
- Yuan, J.; Yu, C.; Peng, J.; Wang, Y.; Ma, J.; Qiu, J.; Li, J.; Zhai, M. Facile synthesis of amphoteric ion exchange membrane by radiation grafting of sodium styrene sulfonate and N, N-dimethylaminoethyl methacrylate for vanadium redox flow battery. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 5194–5202.
- Rajabalizadeh Mojarrad, N.; Sadeghi, S.; Yarar Kaplan, B.m.; Güler, E.; Alkan Gürsel, S. Metal-Salt Enhanced Grafting of Vinylpyridine and Vinylimidazole Monomer Combinations in Radiation Grafted Membranes for High-Temperature PEM Fuel Cells. ACS Appl. Energy Mater. 2019, 3, 532–540.
- Lim, K.L.; Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Selambakkannu, S.; Othman, N.A.F.; Yang, H. Radiation-Grafted Anion-Exchange Membrane for Fuel Cell and Electrolyzer Applications: A Mini Review. Membranes 2021, 11, 397.
- Hoshina, H.; Kasai, N.; Amada, H.; Takahashi, M.; Tanaka, K.; Seko, N. Recovery of scandium from hot spring water with graft adsorbent containing phosphoric groups. J. Ion Exch. 2014, 25, 248–251.
- Hanh, T.T.; Huy, H.T.; Hien, N.Q. Pre-irradiation grafting of acrylonitrile onto chitin for adsorption of arsenic in water. Radiat. Phys. Chem. 2015, 106, 235–241.
- Gao, Q.; Hu, J.; Li, R.; Xing, Z.; Xu, L.; Wang, M.; Guo, X.; Wu, G. Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat. Phys. Chem. 2016, 122, 1–8.
- Sawada, S.-i.; Maekawa, Y. Radiation-Induced Asymmetric Grafting of Different Monomers into Base Films to Prepare Novel Bipolar Membranes. Molecules 2021, 26, 2028.
- Ishihara, R.; Asai, S.; Saito, K. Recent Progress in Charged Polymer Chains Grafted by Radiation-Induced Graft Polymerization; Adsorption of Proteins and Immobilization of Inorganic Precipitates. Quantum Beam Sci. 2020, 4, 20.
- Jaganathan, S.K.; Balaji, A.; Vellayappan, M.V.; Subramanian, A.P.; John, A.A.; Asokan, M.K.; Supriyanto, E. Radiation-induced surface modification of polymers for biomaterial application. J. Mater. Sci. 2015, 50, 2007–2018.
- Chapiro, A. Radiation Chemistry of Polymer System; Interscience: New York, NY, USA, 1962.
- Charlsby, A. Atomic Radiation and Polymers; Pergamon Press: New York, NY, USA, 1960.
- Nasef, M.M.; Güven, O. Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future directions. Prog. Polym. Sci. 2012, 37, 1597–1656.
- Dong, Z.; Wang, Y.; Wen, D.; Peng, J.; Zhao, L.; Zhai, M. Recent Progress in Environmental Applications of Functional Adsorbent Prepared by Radiation techniques: A review. J. Hazard. Mater. 2021, 424, 126887.
- Gubler, L.; Scherer, G.G. Trends for fuel cell membrane development. Desalination 2010, 250, 1034–1037.
- Hoffman, A.S.; Stayton, P.S.; Bulmus, V.; Chen, G.; Chen, J.; Cheung, C.; Chilkoti, A.; Ding, Z.; Dong, L.; Fong, R. Really smart bioconjugates of smart polymers and receptor proteins. J. Biomed. Mater. Res. 2000, 52, 577–586.
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222.
- Hoffman, A.S. Conventional and environmentally-sensitive hydrogels for medical and industrial uses: A review paper. In Polymer Gels; Springer: Berlin/Heidelberg, Germany, 1991; pp. 289–297.
- Carenza, M. Recent achievements in the use of radiation polymerization and grafting for biomedical applications. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1992, 39, 485–493.
- Ulanski, P.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Pietrzak, M.; Stasica, P.; Rosiak, J.M. Polymeric biomaterials synthesized by radiation techniques–current studies at IARC, Poland. Polym. Adv. Technol. 2002, 13, 951–959.
- Rosiak, J.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Stasica, P.; Ulanski, P. Nano-, micro-and macroscopic hydrogels synthesized by radiation technique. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 325–330.
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580.
- Wach, R.A.; Rosiak, J.M.; Ulański, P. Polysaccharides hydrogel-radiation induced formation and medical applications. In Proceedings of the 26th Biomaterials in Medicine and Veterinary Medicine Conference, Rytro, Poland, 12–15 October 2017; Volume 20.
- Yang, J.; Yamato, M.; Kohno, C.; Nishimoto, A.; Sekine, H.; Fukai, F.; Okano, T. Cell sheet engineering: Recreating tissues without biodegradable scaffolds. Biomaterials 2005, 26, 6415–6422.
- Yamato, M.; Akiyama, Y.; Kobayashi, J.; Yang, J.; Kikuchi, A.; Okano, T. Temperature-responsive cell culture surfaces for regenerative medicine with cell sheet engineering. Prog. Polym. Sci. 2007, 32, 1123–1133.
- Pino-Ramos, V.; Meléndez-Ortiz, H.; Ramos-Ballesteros, A.; Bucio, E. Radiation Grafting of Biopolymers and Synthetic Polymers. In Biopolymer Grafting: Applications; Elsevier: Oxford, UK, 2018.
- Alvarez-Lorenzo, C.; Bucio, E.; Burillo, G.; Concheiro, A. Medical devices modified at the surface by γ-ray grafting for drug loading and delivery. Expert Opin. Drug Deliv. 2010, 7, 173–185.
- Singh, B.; Kumar, A. Radiation-induced graft copolymerization of N-vinyl imidazole onto moringa gum polysaccharide for making hydrogels for biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1369–1378.
- García-Vargas, M.; González-Chomón, C.; Magariños, B.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Acrylic polymer-grafted polypropylene sutures for covalent immobilization or reversible adsorption of vancomycin. Int. J. Pharm. 2014, 461, 286–295.
- Tang, Z.; Akiyama, Y.; Okano, T. Recent development of temperature-responsive cell culture surface using poly (N-isopropylacrylamide). J. Polym. Sci. Part B Polym. Phys. 2014, 52, 917–926.
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146.
- Jabbari, E.; Nozari, S. Swelling behavior of acrylic acid hydrogels prepared by γ-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692.
- Costoya, A.; Becerra, L.E.V.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Mayer, C.; Otero, A.; Concheiro, A.; Bucio, E.; Alvarez-Lorenzo, C. Immobilization of antimicrobial and anti-quorum sensing enzymes onto GMA-grafted poly (vinyl chloride) catheters. Int. J. Pharm. 2019, 558, 72–81.
- Zuñiga-Zamorano, I.; Meléndez-Ortiz, H.I.; Costoya, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Poly (vinyl chloride) catheters modified with pH-responsive poly (methacrylic acid) with affinity for antimicrobial agents. Radiat. Phys. Chem. 2018, 142, 107–114.
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327.
- Hiriart-Ramírez, E.; Contreras-García, A.; Garcia-Fernandez, M.J.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Radiation grafting of glycidyl methacrylate onto cotton gauzes for functionalization with cyclodextrins and elution of antimicrobial agents. Cellulose 2012, 19, 2165–2177.
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339.
- Aoki, S.; Fujiwara, K.; Sugo, T.; Suzuki, K. Antimicrobial fabric adsorbed iodine produced by radiation-induced graft polymerization. Radiat. Phys. Chem. 2013, 84, 242–245.
- Riquet, A.-M.; Delattre, J.; Vitrac, O.; Guinault, A. Design of modified plastic surfaces for antimicrobial applications: Impact of ionizing radiation on the physical and mechanical properties of polypropylene. Radiat. Phys. Chem. 2013, 91, 170–179.
- Ping, X.; Wang, M.; Xuewu, G. Surface modification of poly (ethylene terephthalate)(PET) film by gamma-ray induced grafting of poly (acrylic acid) and its application in antibacterial hybrid film. Radiat. Phys. Chem. 2011, 80, 567–572.
- Lim, S.J.; Shin, I.H. Graft copolymerization of GMA and EDMA on PVDF to hydrophilic surface modification by electron beam irradiation. Nucl. Eng. Technol. 2020, 52, 373–380.
- Stasica, P.; Rosiak, J.; Ciach, M.; Radek, M. Approach to construct hydrogel intervertebral disc implants—Experimental and numerical investigations. Eng. Biomater. 2000, 3, 9–14.
- Yoshii, F.; Makuuchi, K.; Sudradjat, A.; Darwis, D.; Razzak, M. Heat stability of radiation crosslinked poly (vinyl alcohol) hydrogel. Ika Kikaigaku 1992, 62, 285–290.
- Darwis, D.; Stasica, P.; Razzak, M.T.; Rosiak, J.M. Characterization of poly (vinyl alcohol) hydrogel for prosthetic intervertebral disc nucleus. Radiat. Phys. Chem. 2002, 63, 539–542.
- Lee, S.-H.; An, S.-J.; Lim, Y.-M.; Huh, J.-B. The efficacy of electron beam irradiated bacterial cellulose membranes as compared with collagen membranes on guided bone regeneration in peri-implant bone defects. Materials 2017, 10, 1018.
- Hidzir, N.M.; Hill, D.J.; Martin, D.; Grøndahl, L. Radiation-induced grafting of acrylic acid onto expanded poly (tetrafluoroethylene) membranes. Polymer 2012, 53, 6063–6071.
- Magaña, H.; Becerra, C.D.; Serrano-Medina, A.; Palomino, K.; Palomino-Vizcaíno, G.; Olivas-Sarabia, A.; Bucio, E.; Cornejo-Bravo, J.M. Radiation Grafting of a Polymeric Prodrug onto Silicone Rubber for Potential Medical/Surgical Procedures. Polymers 2020, 12, 1297.
- Kyomoto, M.; Moro, T.; Saiga, K.; Hashimoto, M.; Ito, H.; Kawaguchi, H.; Takatori, Y.; Ishihara, K. Biomimetic hydration lubrication with various polyelectrolyte layers on cross-linked polyethylene orthopedic bearing materials. Biomaterials 2012, 33, 4451–4459.
- Moro, T.; Kawaguchi, H.; Ishihara, K.; Kyomoto, M.; Karita, T.; Ito, H.; Nakamura, K.; Takatori, Y. Wear resistance of artificial hip joints with poly (2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: Comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials 2009, 30, 2995–3001.
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Concheiro, A.; Jimenez-Paez, V.M.; Bucio, E. Modification of medical grade PVC with N-vinylimidazole to obtain bactericidal surface. Radiat. Phys. Chem. 2016, 119, 37–43.
- Meléndez-Ortiz, H.I.; Alvarez-Lorenzo, C.; Burillo, G.; Magariños, B.; Concheiro, A.; Bucio, E. Radiation-grafting of N-vinylimidazole onto silicone rubber for antimicrobial properties. Radiat. Phys. Chem. 2015, 110, 59–66.
- Valencia-Mora, R.A.; Zavala-Lagunes, E.; Bucio, E. Grafting of thermo-sensitive N-vinylcaprolactam onto silicone rubber through the direct radiation method. Radiat. Phys. Chem. 2016, 124, 155–158.
- Razzak, M.T.; Otsuhata, K.; Tabata, Y.; Ohashi, F.; Takeuchi, A. Modification of natural rubber tubes for biomaterials. II. Radiation-induced grafting of N, N-dimethylaminoethylacrylate (DMAEA) onto natural rubber (NR) tubes. J. Appl. Polym. Sci. 1989, 38, 829–839.
- Razzak, M.T.; Otsuhata, K.; Tabata, Y.; Ohashi, F.; Takeuchi, A. Modification of natural rubber tubes for biomaterials I. Radiation-induced grafting of N, N-dimethyl acrylamide onto natural rubber tubes. J. Appl. Polym. Sci. 1988, 36, 645–653.
- Nowatzki, P.J.; Koepsel, R.R.; Stoodley, P.; Min, K.; Harper, A.; Murata, H.; Donfack, J.; Hortelano, E.R.; Ehrlich, G.D.; Russell, A.J. Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings. Acta Biomater. 2012, 8, 1869–1880.
- Hosny, A.E.-D.M.; Farrag, H.A.; Helmy, O.M.; Hagras, S.A.; El-Hag Ali, A. In-vitro evaluation of antibacterial and antibiofilm efficiency of radiation-modified polyurethane–ZnO nanocomposite to be used as a self-disinfecting catheter. J. Radiat. Res. Appl. Sci. 2020, 13, 215–225.
- Mukherjee, A.; Gupta, B. Radiation-induced graft copolymerization of methacrylic acid onto polypropylene fibers. I. Effect of synthesis conditions. J. Appl. Polym. Sci. 1985, 30, 2643–2653.
- Singh, H.; Tyagi, P. Radiation induced grafting of methacrylic acid onto silk for the immobilization of antimicrobial drug for sustained delivery. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1989, 172, 87–102.
- Tyagi, P.; Gupta, B.; Singh, H. Radiation-induced grafting of 2-hydroxyethyl methacrylate onto polypropylene for biomedical applications. II. Evaluation as antimicrobial suture. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, 30, 303–313.
- Plessier, C.; Gupta, B.; Chapiro, A. Modification of polypropylene fiber by radiation-induced graft copolymerization of acrylonitrile monomer. J. Appl. Polym. Sci. 1998, 69, 1343–1348.
- Gupta, B.; Anjum, N.; Gulrez, S.; Singh, H. Development of antimicrobial polypropylene sutures by graft copolymerization. II. Evaluation of physical properties, drug release, and antimicrobial activity. J. Appl. Polym. Sci. 2007, 103, 3534–3538.
- Yuan, F.; Wei, J.; Tang, E.-Q.; Zhao, K.-Y.; Xue, Y. Synthesis and Modification of Polypropylene by Radiation-induced Grafting. Int. J. Chem. 2009, 1, 75.
- López-Saucedo, F.; Flores-Rojas, G.G.; López-Saucedo, J.; Magariños, B.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Antimicrobial silver-loaded polypropylene sutures modified by radiation-grafting. Eur. Polym. J. 2018, 100, 290–297.
- López-Saucedo, F.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of vinyl monomers separately onto polypropylene monofilament sutures. Radiat. Phys. Chem. 2017, 132, 1–7.
- Marisol Arteaga-Luna, M.; Hugo Pino-Ramos, V.; Magaña, H.; Bucio, E. Polymeric pro-drug sutures for potential local release of salicylic acid. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 792–799.
- López-Saucedo, F.; Flores-Rojas, G.; Bucio, E.; Alvarez-Lorenzo, C.; Concheiro, A.; González-Antonio, O. Achieving antimicrobial activity through poly (N-methylvinylimidazolium) iodide brushes on binary-grafted polypropylene suture threads. MRS Communications 2017, 7, 938–946.
- Tummalapalli, M.; Anjum, S.; Kumari, S.; Gupta, B. Antimicrobial surgical sutures: Recent developments and strategies. Polym. Rev. 2016, 56, 607–630.
- Buchenska, J.; Slomkowski, S.; Tazbir, J.; Sobolewska, E. Antibacterial poly (ethylene terephthalate) yarn containing cephalosporin type antibiotic. Fibres Text. East. Eur. 2003, 11, 41–47.
- Anjum, N.; Gulrez, S.; Singh, H.; Gupta, B. Development of antimicrobial polypropylene sutures by graft polymerization. I. Influence of grafting conditions and characterization. J. Appl. Polym. Sci. 2006, 101, 3895–3901.
- Gupta, B.; Jain, R.; Anjum, N.; Singh, H. Preirradiation grafting of acrylonitrile onto polypropylene monofilament for biomedical applications: I. Influence of synthesis conditions. Radiat. Phys. Chem. 2006, 75, 161–167.
- Gupta, B.; Jain, R.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting onto polypropylene monofilament. Polym. Adv. Technol. 2008, 19, 1698–1703.
- Gupta, B.; Jain, R.; Anjum, N.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting of acrylonitrile onto polypropylene monofilament. III. Hydrolysis of the grafted suture. J. Appl. Polym. Sci. 2004, 94, 2509–2516.
- Jain, R.; Gupta, B.; Anjum, N.; Revagade, N.; Singh, H. Preparation of antimicrobial sutures by preirradiation grafting of acrylonitrile onto polypropylene monofilament. II. mechanical, physical, and thermal characteristics. J. Appl. Polym. Sci. 2004, 93, 1224–1229.
- Wu, M.; Bao, B.; Yoshii, F.; Makuuchi, K. Irradiation of crosslinked, poly (vinyl alcohol) blended hydrogel for wound dressing. J. Radioanal. Nucl. Chem. 2001, 250, 391–395.
- Zhao, L.; Mitomo, H.; Zhai, M.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr. Polym. 2003, 53, 439–446.
- Yang, X.; Liu, Q.; Chen, X.; Yu, F.; Zhu, Z. Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr. Polym. 2008, 73, 401–408.
- Abou Taleb, M.F.; Ismail, S.A.; El-Kelesh, N.A. Radiation synthesis and characterization of polyvinyl alcohol/methacrylic acid–gelatin hydrogel for vitro drug delivery. J. Macromol. Sci. Part A 2008, 46, 170–178.
- Kaur, I.; Bhati, P.; Sharma, S. Radiation induced synthesis of (gelatin-co-PVA)-g-poly (AAc) copolymer as wound dressing material. Adv. Mater. Res. 2014, 3, 183.
- Razzak, M.T.; Darwis, D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113.
- Casimiro, M.; Gil, M.; Leal, J. Suitability of gamma irradiated chitosan based membranes as matrix in drug release system. Int. J. Pharm. 2010, 395, 142–146.
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9.
- Stuart, M.A.C.; Huck, W.T.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113.
- Meléndez-Ortiz, I.H.; Bucio, E. Stimuli-sensitive behaviour of binary graft Co-polymers (PP-g-DMAEMA)-g-NIPAAm and (PP-g-4VP)-g-NIPAAm in acidic and basic medium. Des. Monomers Polym. 2009, 12, 99–108.
- Luk, J.Z.; Cooper-White, J.; Rintoul, L.; Taran, E.; Grøndahl, L. Functionalised polycaprolactone films and 3D scaffolds via gamma irradiation-induced grafting. J. Mater. Chem. B 2013, 1, 4171–4181.
- Rahman, M.; Sreearunothai, P.; Opaprakasit, P. Development and Characterization of Photoinduced Acrylamide-Grafted Polylactide Films for Biomedical Applications. Int. J. Polym. Sci. 2017, 2017, 5651398.
- Casimiro, M.H.; Gomes, S.R.; Rodrigues, G.; Leal, J.P.; Ferreira, L.M. Chitosan/Poly (vinylpyrrolidone) matrices obtained by gamma-irradiation for skin scaffolds: Characterization and preliminary cell response studies. Materials 2018, 11, 2535.
- Casimiro, M.H.; Lancastre, J.J.; Rodrigues, A.P.; Gomes, S.R.; Rodrigues, G.; Ferreira, L.M. Chitosan-Based matrices prepared by gamma irradiation for tissue regeneration: Structural properties vs. preparation method. In Applications of Radiation Chemistry in the Fields of Industry, Biotechnology and Environment; Springer: Berlin/Heidelberg, Germany, 2017; pp. 121–145.
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-responsive polymer modified surface for cell sheet engineering. Polymers 2012, 4, 1478–1498.
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010, 267, 54–70.
- Takahashi, H.; Nakayama, M.; Yamato, M.; Okano, T. Controlled chain length and graft density of thermoresponsive polymer brushes for optimizing cell sheet harvest. Biomacromolecules 2010, 11, 1991–1999.
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Okano, T. Scaffold-free tissue engineering using cell sheet technology. RSC Adv. 2012, 2, 2184–2190.
- Riquet, A.; Rohman, G.; Guinault, A.; Demilly, M. Surface modification of polypropylene by radiation grafting of hydrophilic monomers: Physicochemical properties. Surf. Eng. 2011, 27, 234–241.
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Die Makromol. Chem. Rapid Commun. 1990, 11, 571–576.
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials 2003, 24, 2309–2316.
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly (N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511.
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Maeda, N.; Watanabe, H.; Yamamoto, K.; Nagai, S.; Kikuchi, A.; Tano, Y. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 2004, 77, 379–385.
- Fukumori, K.; Akiyama, Y.; Yamato, M.; Kobayashi, J.; Sakai, K.; Okano, T. Temperature-responsive glass coverslips with an ultrathin poly (N-isopropylacrylamide) layer. Acta Biomater. 2009, 5, 470–476.
- Akiyama, Y.; Yamato, M.; Okano, T. Preparation of poly (N-isopropylacrylamide) grafted polydimethylsiloxane by using electron beam irradiation. J. Robot. Mechatron. 2013, 25, 631–636.
- Kumar, P.A.; Sreenivasan, K.; Kumary, T. Alternate method for grafting thermoresponsive polymer for transferring in vitro cell sheet structures. J. Appl. Polym. Sci. 2007, 105, 2245–2251.
- von Recum, H.; Okano, T.; Kim, S.W. Growth factor release from thermally reversible tissue culture substrates. J. Control. Release 1998, 55, 121–130.
- Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Two-dimensional manipulation of cardiac myocyte sheets utilizing temperature-responsive culture dishes augments the pulsatile amplitude. Tissue Eng. 2001, 7, 141–151.
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. 2010, 11, 014111.
- Jeong, S.I.; Park, S.-C.; Park, S.-J.; Kim, E.-J.; Heo, H.; Park, J.-S.; Gwon, H.-J.; Lim, Y.-M.; Jang, M.-K. One-step synthesis of gene carrier via gamma irradiation and its application in tumor gene therapy. Int. J. Nanomed. 2018, 13, 525.
- Turmanova, S.; Trifonov, A.; Kalaijiev, O.; Kostov, G. Radiation grafting of acrylic acid onto polytetrafluoroethylene films for glucose oxidase immobilization and its application in membrane biosensor. J. Membr. Sci. 1997, 127, 1–7.
- Muñoz-Muñoz, F.; Ruiz, J.-C.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Novel interpenetrating smart polymer networks grafted onto polypropylene by gamma radiation for loading and delivery of vancomycin. Eur. Polym. J. 2009, 45, 1859–1867.
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605.
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107.
- Demirdirek, B.; Uhrich, K.E. Novel salicylic acid-based chemically crosslinked pH-sensitive hydrogels as potential drug delivery systems. Int. J. Pharm. 2017, 528, 406–415.
- Vázquez-González, B.; Meléndez-Ortiz, H.I.; Díaz-Gómez, L.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Silicone rubber modified with methacrylic acid to host antiseptic drugs. Macromol. Mater. Eng. 2014, 299, 1240–1250.
- Kayal, S. Thermoresponsive magnetic/polymer composite nanoparticles for biomedical applications. Mater. Today Proc. 2021, 41, 1116–1119.
- Mutalik, S.; Suthar, N.A.; Managuli, R.S.; Shetty, P.K.; Avadhani, K.; Kalthur, G.; Kulkarni, R.V.; Thomas, R. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int. J. Biol. Macromol. 2016, 86, 709–720.
- Magaña, H.; Palomino, K.; Cornejo-Bravo, J.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Radiation-grafting of acrylamide onto silicone rubber films for diclofenac delivery. Radiat. Phys. Chem. 2015, 107, 164–170.
- Melendez-Ortiz, H.I.; Díaz-Rodríguez, P.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Binary graft modification of polypropylene for anti-inflammatory drug–device combo products. J. Pharm. Sci. 2014, 103, 1269–1277.
- De Oliveira, M.; Parra, D.; Amato, V.; Lugão, A. Hydrogel membranes of PVAl/clay by gamma radiation. Radiat. Phys. Chem. 2013, 84, 111–114.
- Gagliardi, M. In vitro haematic proteins adsorption and cytocompatibility study on acrylic copolymer to realise coatings for drug-eluting stents. Mater. Sci. Eng. C 2012, 32, 2445–2451.
- Burillo, G.; Bucio, E.; Arenas, E.; Lopez, G.P. Temperature and pH-sensitive swelling behavior of binary DMAEMA/4VP grafts on poly (propylene) films. Macromol. Mater. Eng. 2007, 292, 214–219.
- Bucio, E.; Burillo, G. Radiation grafting of pH and thermosensitive N-isopropylacrylamide and acrylic acid onto PTFE films by two-steps process. Radiat. Phys. Chem. 2007, 76, 1724–1727.
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 434–438.
- Casimiro, M.; Gil, M.; Leal, J. Drug release assays from new chitosan/pHEMA membranes obtained by gamma irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 406–409.
- Ramírez-Fuentes, Y.S.; Bucio, E.; Burillo, G. Radiation-induced grafting of N-isopropylacrylamide and acrylic acid onto polypropylene films by two step method. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 265, 183–186.
- Ikram, S.; Kumari, M.; Gupta, B. Thermosensitive membranes by radiation-induced graft polymerization of N-isopropyl acrylamide/acrylic acid on polypropylene nonwoven fabric. Radiat. Phys. Chem. 2011, 80, 50–56.
- Contreras-García, A.; Alvarez-Lorenzo, C.; Taboada, C.; Concheiro, A.; Bucio, E. Stimuli–responsive networks grafted onto polypropylene for the sustained delivery of NSAIDs. Acta Biomater. 2011, 7, 996–1008.
- Piao, M.-H.; Yang, D.-S.; Yoon, K.-R.; Lee, S.-H.; Choi, S.-H. Development of an electrogenerated chemiluminescence biosensor using carboxylic acid-functionalized MWCNT and Au nanoparticles. Sensors 2009, 9, 1662–1677.
- Yang, J.-H.; Lee, J.-C.; Choi, S.-H. Tyrosinase-immobilized biosensor based on the functionalized hydroxyl group-MWNT and detection of phenolic compounds in red wines. J. Sens. 2009, 2009, 916515.
- Yang, D.-S.; Jung, D.-J.; Choi, S.-H. One-step functionalization of multi-walled carbon nanotubes by radiation-induced graft polymerization and their application as enzyme-free biosensors. Radiat. Phys. Chem. 2010, 79, 434–440.
- Kim, S.-K.; Kwen, H.-D.; Choi, S.-H. Fabrication of a microbial biosensor based on QD-MWNT supports by a one-step radiation reaction and detection of phenolic compounds in red wines. Sensors 2011, 11, 2001–2012.
- Kim, K.-I.; Kang, H.-Y.; Lee, J.-C.; Choi, S.-H. Fabrication of a multi-walled nanotube (MWNT) ionic liquid electrode and its application for sensing phenolics in red wines. Sensors 2009, 9, 6701–6714.
- Lee, Y.-J.; Chung, D.-J.; Oh, S.-H.; Choi, S.-H. Introduction of bifunctional group onto MWNT by radiation-induced graft polymerization and its use as biosensor-supporting materials. J. Nanomater. 2012, 2012, 127613.
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules 1993, 26, 2496–2500.
- Pino-Ramos, V.H.; Flores-Rojas, G.G.; Alvarez-Lorenzo, C.; Concheiro, A.; Bucio, E. Graft copolymerization by ionization radiation, characterization, and enzymatic activity of temperature-responsive SR-g-PNVCL loaded with lysozyme. React. Funct. Polym. 2018, 126, 74–82.
- Abd Halin, N.I.; Al-Khatib, M.F.R.; Salleh, H.M.; Nasef, M.M. Preparation and Candida rugosa Lipase Immobilization on Nylon-6 Grafted and Aminated (Polyvinyl Benzyl Chloride) Microfibers. Bull. Chem. React. Eng. Catal. 2019, 14, 369–379.
- Alkhatib, M.; Bahrudin, N.A.; SALLEH, H.M.; Nasef, M.M.; Ting, T.M. Lipase immobilization on fibers grafted with polyglycidyl methachrylate. IIUM Eng. J. 2019, 20, 12–23.
- Kamal, H.; Sabry, G.M.; Lotfy, S.; Abdallah, N.M.; Rosiak, J.; Hegazy, E.s.A. Immobilization of glucoamylase on polypropylene fibers modified by radiation induced graft copolymerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 45, 65–75.
- Garnett, J.; Jankiewicz, S.; Levot, R.; Sangster, D. Insolubilisation of biologically active materials with novel radiation graft copolymers. Radiat. Phys. Chem. 1985, 25, 509–516.
- Dong, L.C.; Hoffman, A.S. Thermally reversible hydrogels: III. Immobilization of enzymes for feedback reaction control. J. Control. Release 1986, 4, 223–227.
- Dong, L.C.; Hoffman, A.S. A new method for immobilization of biomolecules using preirradiation grafting at low temperature. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1986, 28, 177–182.
- Hongfei, H.; Guanghui, W.; Jilan, W. Immobilization of peroxidase on SPEU film via radiation grafting. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1988, 31, 761–767.
- Schmidt, M.; Prager, A.; Schönherr, N.; Gläser, R.; Schulze, A. Reagent-free immobilization of industrial lipases to develop lipolytic membranes with self-cleaning surfaces. Membranes 2022, 12, 599.
- Xu, C.; Huang, W.; Zhou, Y.; Yan, D.; Chen, S.; Huang, H. Graft copolymerization of N-vinyl-2-pyrrolidone onto pre-irradiated poly (vinylidene fluoride) powder. Radiat. Phys. Chem. 2012, 81, 426–431.
- Qin, Q.; Hou, Z.; Lu, X.; Bian, X.; Chen, L.; Shen, L.; Wang, S. Microfiltration membranes prepared from poly (N-vinyl-2-pyrrolidone) grafted poly (vinylidene fluoride) synthesized by simultaneous irradiation. J. Membr. Sci. 2013, 427, 303–310.
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (PVDF) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 2721.
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Zhu, B.-K. Modification of polytetrafluoroethylene porous membranes by electron beam initiated surface grafting of binary monomers. J. Membr. Sci. 2009, 339, 33–38.
- Deng, B.; Yang, X.; Xie, L.; Li, J.; Hou, Z.; Yao, S.; Liang, G.; Sheng, K.; Huang, Q. Microfiltration membranes with pH dependent property prepared from poly (methacrylic acid) grafted polyethersulfone powder. J. Membr. Sci. 2009, 330, 363–368.
- Deng, B.; Li, J.; Hou, Z.; Yao, S.; Shi, L.; Liang, G.; Sheng, K. Microfiltration membranes prepared from polyethersulfone powder grafted with acrylic acid by simultaneous irradiation and their pH dependence. Radiat. Phys. Chem. 2008, 77, 898–906.
- Mok, S.; Worsfold, D.; Fouda, A.; Matsuura, T. Surface modification of polyethersulfone hollow-fiber membranes by γ-ray irradiation. J. Appl. Polym. Sci. 1994, 51, 193–199.
- Van der Bruggen, B. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. Sci. 2009, 114, 630–642.
- Schmidt, M.; Zahn, S.; Gehlhaar, F.; Prager, A.; Griebel, J.; Kahnt, A.; Knolle, W.; Konieczny, R.; Gläser, R.; Schulze, A. Radiation-Induced Graft Immobilization (RIGI): Covalent Binding of Non-Vinyl Compounds on Polymer Membranes. Polymers 2021, 13, 1849.
- Adem, E.; Avalos-Borja, M.; Bucio, E.; Burillo, G.; Castillon, F.; Cota, L. Surface characterization of binary grafting of AAc/NIPAAm onto poly (tetrafluoroethylene)(PTFE). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 234, 471–476.
- Casimiro, M.; Leal, J.; Gil, M. Characterisation of gamma irradiated chitosan/pHEMA membranes for biomedical purposes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 236, 482–487.
- He, C.; Gu, Z. Studies on acrylic acid–grafted polyester fabrics by electron beam preirradiation method. I. Effects of process parameters on graft ratio and characterization of grafting products. J. Appl. Polym. Sci. 2003, 89, 3931–3938.
- Park, J.S.; Kim, J.H.; Nho, Y.C.; Kwon, O.H. Antibacterial activities of acrylic acid-grafted polypropylene fabric and its metallic salt. J. Appl. Polym. Sci. 1998, 69, 2213–2220.
- Hassan, M.S.; Ibrahim, H.M. Characterization and antimicrobial properties of metal complexes of polypropylene fibers grafted with acrylic acid using gamma irradiation. Polym. Adv. Technol. 2016, 27, 532–541.
- Montoya-Villegas, K.A.; Ramírez-Jiménez, A.; Licea-Claverie, Á.; Pérez-Sicairos, S.; Bucio, E.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials 2019, 12, 3284.
- Kumar, V.; Bhardwaj, Y.; Rawat, K.; Sabharwal, S. Radiation-induced grafting of vinylbenzyltrimethylammonium chloride (VBT) onto cotton fabric and study of its anti-bacterial activities. Radiat. Phys. Chem. 2005, 73, 175–182.
- Flores-Rojas, G.; López-Saucedo, F.; Vázquez, E.; Hernández-Mecinas, E.; Huerta, L.; Cedillo, G.; Concheiro, A.; Alvarez-Lorenzo, C.; Bucio, E. Synthesis of cellulose microfilms grafted with N-vinylcaprolactam using gamma-rays and loading of antimicrobial drugs. Cellulose 2020, 27, 2785–2801.
- Huang, K.-S.; Yang, C.-H.; Huang, S.-L.; Chen, C.-Y.; Lu, Y.-Y.; Lin, Y.-S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578.
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial approaches for textiles: From research to market. Materials 2016, 9, 498.
- Seino, S.; Imoto, Y.; Kitagawa, D.; Kubo, Y.; Kosaka, T.; Kojima, T.; Nitani, H.; Nakagawa, T.; Yamamoto, T.A. Radiochemical synthesis of silver nanoparticles onto textile fabrics and their antibacterial activity. J. Nucl. Sci. Technol. 2016, 53, 1021–1027.
- Ferraz, C.C.; Varca, G.H.; Ruiz, J.-C.; Lopes, P.S.; Mathor, M.B.; Lugão, A.B.; Bucio, E. Radiation-grafting of thermo-and pH-responsive poly (N-vinylcaprolactam-co-acrylic acid) onto silicone rubber and polypropylene films for biomedical purposes. Radiat. Phys. Chem. 2014, 97, 298–303.
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S.J. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266.
- Goel, N.; Kumar, V.; Rao, M.; Bhardwaj, Y.; Sabharwal, S. Functionalization of cotton fabrics by radiation induced grafting of quaternary salt to impart antibacterial property. Radiat. Phys. Chem. 2011, 80, 1233–1241.
- Yang, J.M.; Lin, H.T.; Wu, T.H.; Chen, C.C. Wettability and antibacterial assessment of chitosan containing radiation-induced graft nonwoven fabric of polypropylene-g-acrylic acid. J. Appl. Polym. Sci. 2003, 90, 1331–1336.
- Terada, A.; Yuasa, A.; Tsuneda, S.; Hirata, A.; Katakai, A.; Tamada, M. Elucidation of dominant effect on initial bacterial adhesion onto polymer surfaces prepared by radiation-induced graft polymerization. Colloids Surf. B. Biointerfaces 2005, 43, 99–107.
- Murata, H.; Koepsel, R.R.; Matyjaszewski, K.; Russell, A.J. Permanent, non-leaching antibacterial surfaces—2: How high density cationic surfaces kill bacterial cells. Biomaterials 2007, 28, 4870–4879.
- Anjum, N.; Bellon-Fontaine, M.N.; Herry, J.M.; Riquet, A.M. A novel process to develop modified polymeric surfaces for the analysis of bacterial adhesion: Surface properties and adhesion test. J. Appl. Polym. Sci. 2008, 109, 1746–1756.
- Huang, C.; Wang, H.; Xu, Y.H. Functional finishing on silk fabric with acrylamide monomer and chitosan. Adv. Mater. Res. 2011, 175–176, 696–702.
- Ye, F.; Huang, C.; Jiang, X.; He, W.; Gao, X.; Ma, L.; Ao, J.; Xu, L.; Wang, Z.; Li, Q. Reusable fibrous adsorbent prepared via Co-radiation induced graft polymerization for iodine adsorption. Ecotoxicol. Environ. Saf. 2020, 203, 111021.
- Goel, N.; Rao, M.; Kumar, V.; Bhardwaj, Y.; Chaudhari, C.; Dubey, K.; Sabharwal, S. Synthesis of antibacterial cotton fabric by radiation-induced grafting of trimethylammonium chloride (MAETC) onto cotton. Radiat. Phys. Chem. 2009, 78, 399–406.
- Salmieri, S.; Khan, R.A.; Safrany, A.; Lacroix, M. Gamma rays-induced 2-hydroxyethyl methacrylate graft copolymerization on methylcellulose-based films: Structure analysis and physicochemical properties. Ind. Crops Prod. 2015, 70, 64–71.
This entry is offline, you can click here to edit this entry!
