Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Leptospirosis is one of the most widespread and dangerous zoonoses in the world. The main cause of death in leptospirosis is the development of a severe form of leptospirosis—Weil’s disease, which affects the kidneys, lungs, and liver, which are a “triad” of target organs in leptospirosis.
- leptospirosis
- dysbiosis
- Weil’s disease
1. Introduction
Leptospirosis is a re-emerging zoonosis, caused by Leptospira spp. It is estimated to infect more than a million people with approximately 60,000 deaths annually [1]. Disease is usually transmitted by contact with the urine of a host carrying the pathogen in water or soil, causing infection in humans through the skin or the gastrointestinal tract (GI) [2]. Human leptospirosis has a wide range of clinical manifestations, including mild, asymptomatic infections, as well as serious and life-threatening complications with multi-organ dysfunction, e.g., when kidneys, lungs, and liver are seriously damaged [3][4]. Weil’s syndrome (10% of cases), is a severe form of leptospirosis with a high mortality rate; it is characterized by hepatic dysfunctions associated with renal failure and hemorrhages [5]. Severe leptospirosis patients should receive early recognition and intensive medical care. The pathology of leptospirosis and the factors that cause severe leptospirosis are currently unclear [6][7] and antibiotic therapy is still the preferred treatment. However, due to the difficulty of early diagnosis of leptospirosis, some patients infected with L. interrogans often develop multiorgan dysfunction [8].
Both host factors and pathogens may play an important role in the pathogenesis of leptospirosis [9]. Induction of an inflammatory response by a pathogen can initiate destructive immune mechanisms leading to host tissue damage, sepsis, and death [10]. The extensive release of cytokines, including interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), is known as a cytokine storm. Several studies have shown how cytokines contribute to pathogenesis and clinical manifestations of leptospirosis [11][12].
Gut dysbiosis is one of the potential factors that can affect the clinical course of leptospirosis [13]. Gut microbiota interacts with the host immune system in ways that influence the development of different diseases [14]. The gut microbiota generates a wide range of metabolites, such as short-chain fatty acids (SCFAs) or secondary bile acids [15]. SCFA has anti-inflammatory effects by inducing apoptosis and preserving the mucosal barriers to endotoxin infiltration [16]. There is growing evidence that there is a critical link between the gut microbiome and other organs most affected by leptospirosis, such as the liver, lungs, and kidneys [17][18].
2. Leptospira Immunity
The innate immune system is the first line of host defense and is essential in the early recognition and elimination of leptospires [9]. Microbial Pathogen-Associated Molecular Patterns (PAMPs) will be recognized by the Pattern Recognition Receptors (PRRs) expressed at the surface of innate immune cells, such as macrophages and dendritic cells (DCs), mainly the Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [19]. Among TLRs, TLR2 and TLR4 are the most studied in leptospirosis now. TLR4 can be activated by Lipopolysaccharides (LPS) from Gram-negative bacteria, which causes a pro-inflammatory cytokine- and chemokine-dependent response [20]. Leptospiral LPS is less endotoxic than Gram-negative LPS and activates human macrophages via TLR2 rather than TLR4 [21] (Figure 1).
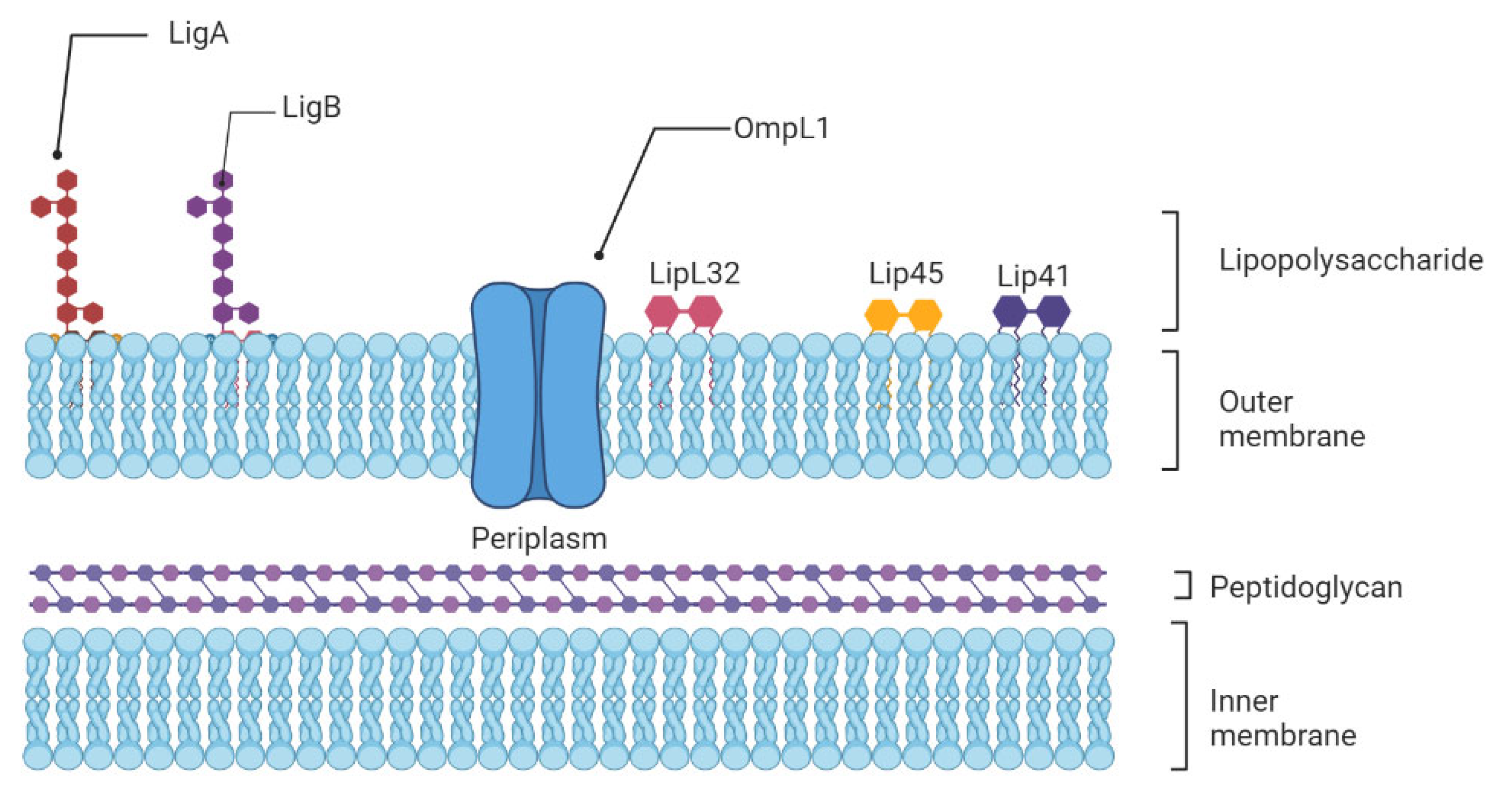
Figure 1. Schematic representation of the architecture of the leptospiral membrane. The inner membrane is closely associated with the peptidoglycan cell wall, which is overlaid by the outer membrane. Surface-exposed lipoproteins (LipL32, LigA, LigB), the transmembrane outer membrane protein porin L1 (OmpL1), and lipopolysaccharide are among the main components of the outer membrane.
This different recognition is attributed to the unusual composition of the leptospiral Lipid A moiety and could be a strategy that pathogenic Leptospira may use to avoid activation of immune cells, contributing to the initiation of the disease in humans [22]. In mice, which are resistant to leptospirosis, the LPS is recognized by both TLR2 and TLR4. Indeed, both TLR4 and TLR2 stimulation is important in controlling leptospirosis in mice. Infected with L. interrogans, double TLR2/TLR4 knockout mice died quickly from hepatic and renal failure [23]. Other receptors may mediate the binding of pathogens to host cells before their ingestion by phagocytic cells. DCs express diverse PRRs, including C-type lectins as DC-specific intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN), an endocytic receptor that recognizes high-mannose glycans and fucose-containing antigens on pathogens’ surfaces [24]. As polymorphonuclear neutrophils (PMNs) constitute the largest population of intravascular phagocytes, they are expected to play a critical role in leptospiral clearance. Neutrophils are major phagocytic cells that utilize a combination of reactive oxygen species (ROS), cytotoxic granules, antimicrobial peptides, and Neutrophil Extracellular Traps (NETs) to kill and degrade the invading pathogen [25].
The PAMPs/PRR association triggers an inflammatory cascade by activating multiple intracellular signaling pathways, including the NF-κB and activator protein 1 (AP-1) transcription factors, which in turn regulates the expression of cytokines, prostaglandins (PGs), and nitric oxide (NO) [26][27]. PGs and NO are pro-inflammatory molecules that enhance arterial dilatation and vascular permeability, both of which are essential for immune cell influx [28][29]. Pro-inflammatory cytokines include interleukins (IL)-1β, IL-6, IL-12, interferons (IFNs), and tumor necrosis factors (TNFs), as well as chemokines, which act as chemoattractants to recruit leucocytes to the site of tissue damage and infection [30]. In addition, activation of the complement system alternative route, particularly during the early hours of infection, is an essential aspect of the innate immune system and elimination of leptospires [31]. For example, saprophytic L. biflexa is killed within a few minutes in the presence of normal human serum in vitro, but it is not true for pathogenic Leptospira species which can survive, and are more resistant to the action of the complement system, especially if they are virulent [32]. Considering that immature DCs express TLR2 and that leptospires are able to interact with this receptor in human macrophages, it is particularly relevant to investigate the cross-talk between DC-SIGN and TLR pathways in DCs [33]. Therefore, further investigations on DCs activation and antigen presentation to T cells are required to elucidate the establishment of adaptive immune responses in leptospirosis.
3. Cytokines, Chemokines, Genetics, and Their Role in Severe Leptospirosis
Inflammatory cytokines and cytokine regulators participate in pathogen clearance without excessive inflammation-induced organ damage. Severe infectious diseases are often associated with a prolonged increase in pro-inflammatory IL-1β, TNF-α, IL-6 expression, or “cytokine storm”, causing persistent inflammation and followed by a massive and systemic production of anti-inflammatory cytokines [34]. Interestingly, the clinical hallmarks of severe leptospirosis can resemble septic shock, with multi-organ failure, hypotension, and death, which suggest that the development of severe leptospirosis could be associated with dysregulated inflammation [35]. There have been data obtained during clinical studies that confirm this hypothesis. The very first research of cytokines in human leptospirosis showed a significant increase in TNF-α levels from patient sera [36][37]. However, as TNF-α is an early phase cytokine, its levels are reduced or undetectable with disease progression [9]. Further investigations reported higher production of cytokines in severe compared to mild disease [10][11]. Serum levels of pro-inflammatory IL-6, chemokine IL-8, and anti-inflammatory IL-10 were significantly higher among patients that developed SPHS compared to non-SPHS leptospirosis patients [10]. In addition, concentrations of these cytokines were elevated in sera from patients with organ dysfunction compared to mild cases without organ involvement [38]. Additionally, in human leptospirosis, contradictory findings showed either greater IL-10 levels or no significant change in IL-10 levels [10][39]. Inflammatory response is restricted by regulatory cytokines, such as IL-4 or IL-13, promoting T helper (Th) lymphocyte differentiation toward Th2, suppressing tissue-damaging effects of sustained inflammation [40]. Serum levels of IL-4 were increased in human patients and frequencies in IL-4 and IL-4R receptor gene polymorphisms were significantly higher in leptospirosis patients compared to healthy subjects [41].
High levels of chemokines are found in susceptible hamsters, and are associated with organ damage and poor outcome [10][42]. Notably, patients with severe clinical signs had higher levels of CXCL8/IL-8 expression, and these patients had a higher mortality rate [39]. In addition, high concentrations of adhesion molecules (ICAM-1 or VCAM) are associated with leptospirosis-induced organ damage [43]. Chemokines along with endothelial adhesion molecules are induced by TNF-α, which promotes leukocyte attraction and extravasation into injured tissue.
Pentraxins are a class of evolutionarily conserved proteins that trace their evolutionary roots back to early invertebrates. C-reactive protein (CRP) and serum amyloid P component (SAP) are short pentraxins, and Pentraxin 3 (PTX-3) is a long one. In leptospirosis patients, increasing serum levels of PTX-3 were associated with mortality and disease severity [44].
The study of genetic susceptibility to infectious illness has experienced a revolutionary transformation in the previous decade [45]. Variations in the genetic makeup of the host may result in changes in susceptibility to leptospirosis [46]. Human leukocyte antigen (HLA), cytokine genes, and killer-cell immunoglobulin-like receptors (KIR) polymorphisms were studied in leptospirosis patients and healthy controls [41]. According to the researchers, susceptibility to leptospiral infection was significantly associated with alleles in the HLA-A and B loci and various HLA haplotypes. Patients with a history of leptospirosis exhibited considerably more polymorphisms in the IL-4 and IL-4R genes. Other researchers, on the other hand, discovered that leptospirosis infection was responsive to genetic diversity in the IL12RB1, IL1, and CISH genes [47]. TLR1 Ile602Ser and TLR2 Arg753Gln gene polymorphisms have also been found to substantially influence the development of severe leptospirosis with jaundice and hepatic insufficiency [48].
4. Immune Evasion in Leptospirosis
4.1. Neutrophils Are Poor Phagocytes
Neutrophils are key cells that act against extracellular pathogens through three major mechanisms, i.e., phagocytosis, degranulation, and the release of extracellular traps (Figure 2). According to a recent study, most pathogenic leptospires were found on the neutrophil surface and were not phagocytized. Saprophytic leptospires, on the other hand, were taken up. Intracellular ROS levels were found to be related to leptospire uptake. Overall, pathogenic leptospires appear to be able to avoid or significantly reduce their uptake by human neutrophils, but the precise mechanisms and in vivo relevance involved are unknown [49].
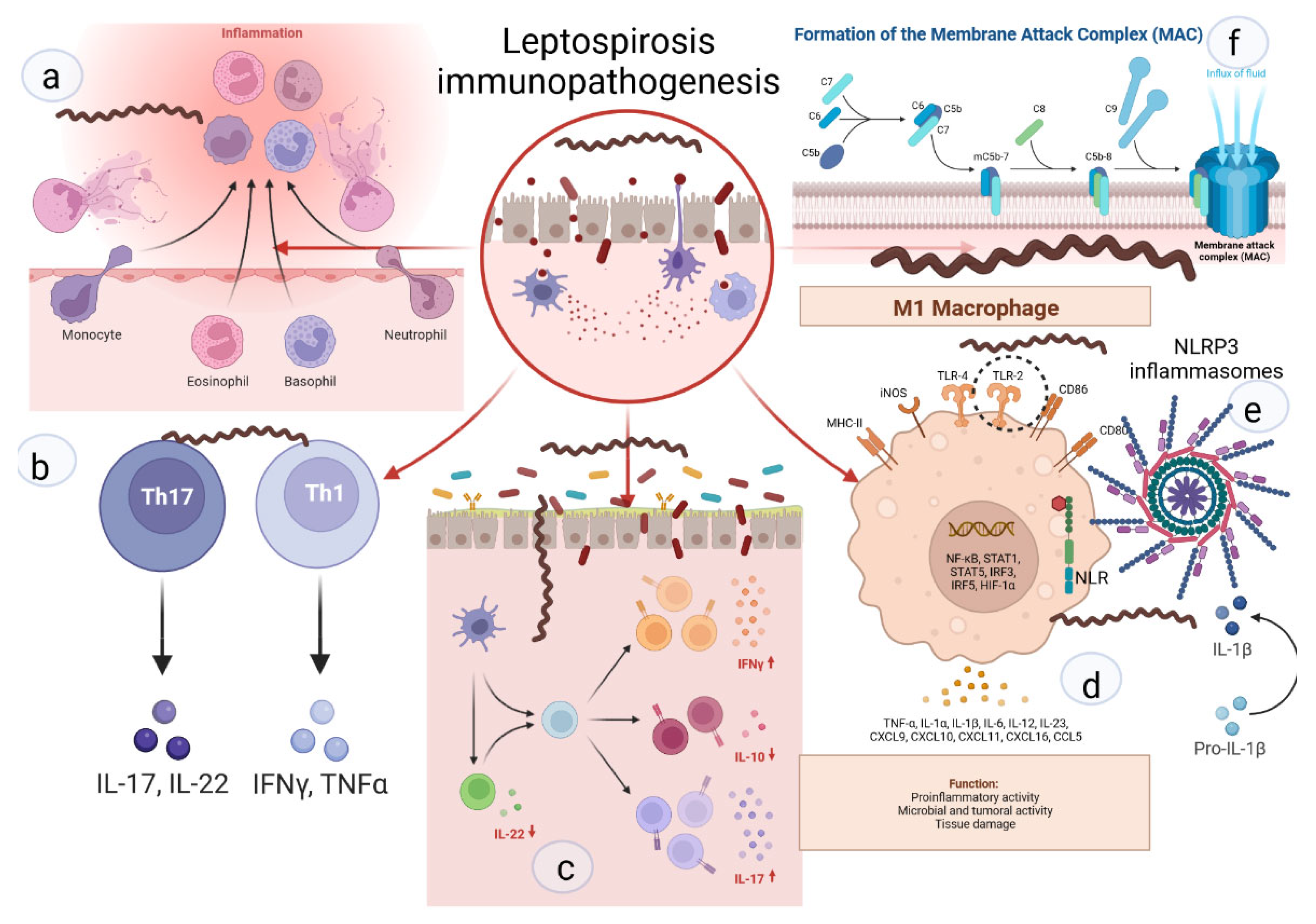
Figure 2. Key links in the immunopathogenesis of leptospirosis: (a) recruitment of neutrophils to the inflammatory zone and formation of NETs; (b) activation of differentiation of pro-inflammatory subpopulations of T-helpers; (c) increase in pro-inflammatory signaling against the background of deficiency of suppressor signals and Treg cells; (d) activation of M1-macrophages, stimulation of TLR2; (e) NLR with subsequent formation of NLRP3-inflammasome and subsequent maturation of IL1b; (f) -formation of MAC and destruction of the cell membrane.
Regarding degranulation, it has been shown that both virulent and avirulent Leptospira spp. are killed by both primary and secondary granule contents. The primary (azurophilic) granules, which contain myeloperoxidase (MPO), heparin-binding protein (HBP), defensins, and other antimicrobial peptides (AMPs), showed the highest microbicidal activity [50]. Remarkably, it has been recently shown that the L. interrogans outer membrane protein LipL21 is a potent inhibitor of neutrophil MPO [51]. This heme-containing peroxidase enzyme, which is primarily expressed in neutrophils, catalyzes the formation of ROS intermediates in the presence of hydrogen peroxide and halides such as hypochlorous acid (HOCl), a major effector of neutrophil-mediated microbial killing.
In regard to the NET formation, it was shown that Leptospira spp. could induce NET release in human neutrophils and that bacterial number, pathogenicity, and viability were important factors in NET release induction, whereas bacterial motility was not [52]. Interestingly, although NETs reduced leptospire survival, pathogenic but not saprophytic Leptospira spp. displayed nuclease activity and damaged DNA, suggesting that pathogenic leptospires may counteract this microbicidal mechanism [53].
4.2. Leptospiral Complement Evasion
Although leptospiral serum resistance to host complement was described many decades ago, the mechanisms underlying this resistance have only recently begun to be unraveled. Pathogens have developed complex strategies to avoid the immunological defense systems of a variety of hosts, including mechanisms to avoid complement activation and/or lytic complement attack. Among these mechanisms are the acquisition of host fluid-phase complement regulators, notably Factor H and C4b-binding protein (C4BP), the secretion of proteases that inactivate key complement components, and the expression of proteins in the pathogen surface that may inhibit or modulate complement activation [54]. The multifunctional LigB protein also binds to C3b and C4b and interferes with complement activation [55]. Lsa30, a novel leptospiral adhesion protein, may help pathogenic Leptospira to escape the immune system by interfering with the complement cascade through interaction with the C4bp regulator [56]. Lsa33 also binds to C4bp and may be important in immune evasion [57]. The recently described leptospiral complement regulator-acquiring protein A (LcpA) also binds to C4bp [58].
This entry is adapted from the peer-reviewed paper 10.3390/biom12121830
References
- Govindan, P.; Pitchaikani, S.; Kandasamy, S.; Rajan, M.; Shakila, H.; Eed, E.M.; Elfasakhany, A.; Pugazhendhi, A. Biomacromolecules of chitosan—Bacopa saponin based LipL32 gene delivery system for leptospirosis therapy. Environ. Res. 2021, 202, 111699.
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009, 7, 736–747.
- Latchoumi, T.; Reddy, M.S.; Balamurugan, K. Applied machine learning predictive analytics to SQL injection attack detection and prevention. Eur. J. Mol. Clin. Med. 2020, 7, 2020.
- Abdullah, M.; Chaubey, K.K.; Namdev, R.; Sharma, R. Leptospira: A Review on Pathogenesis and Host Immune Response. Ann. Rom. Soc. Cell Biol. 2021, 25, 18686–18694.
- Haake, D.A.; Levett, P.N. Leptospirosis in Humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97.
- Limothai, U.; Lumlertgul, N.; Sirivongrangson, P.; Kulvichit, W.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Peerapornratana, S.; Chirathaworn, C.; Praditpornsilpa, K.; et al. The role of leptospiremia and specific immune response in severe leptospirosis. Sci. Rep. 2021, 11, 14630.
- Petakh, P.; Isevych, V.; Griga, V.; Kamyshnyi, A. The risk factors of severe leptospirosis in the Transcarpathian region of Ukraine–search for “red flags”. Arch. Balk Med. Union 2022, 57, 231–237.
- Jiménez, J.I.S.; Marroquin, J.L.H.; Richards, G.A.; Amin, P. Leptospirosis: Report from the task force on tropical diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2018, 43, 361–365.
- Fraga, T.R.; Barbosa, A.S.; Isaac, L. Leptospirosis: Aspects of Innate Immunity, Immunopathogenesis and Immune Evasion From the Complement System. Scand. J. Immunol. 2011, 73, 408–419.
- Reis, E.A.G.; Hagan, J.; Ribeiro, G.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Montgomery, R.; Shaw, A.C.; Ko, A.; Reis, M.G. Cytokine Response Signatures in Disease Progression and Development of Severe Clinical Outcomes for Leptospirosis. PLoS Negl. Trop. Dis. 2013, 7, e2457.
- Mikulski, M.; Boisier, P.; Lacassin, F.; Soupé-Gilbert, M.-E.; Mauron, C.; Bruyère-Ostells, L.; Bonte, D.; Barguil, Y.; Gourinat, A.-C.; Matsui, M.; et al. Severity markers in severe leptospirosis: A cohort study. Eur. J. Clin. Microbiol. 2015, 34, 687–695.
- Kyriakidis, I.; Samara, P.; Papa, A. Serum TNF-α, sTNFR1, IL-6, IL-8 and IL-10 levels in Weil’s syndrome. Cytokine 2011, 54, 117–120.
- Xie, X.; Liu, J.; Chen, X.; Zhang, S.; Tang, R.; Wu, X.; Zhang, W.; Cao, Y. Gut microbiota involved in leptospiral infections. ISME J. 2022, 16, 764–773.
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95.
- Bruning, J.; Chapp, A.; Kaurala, G.A.; Wang, R.; Techtmann, S.; Chen, Q.-H. Gut Microbiota and Short Chain Fatty Acids: Influence on the Autonomic Nervous System. Neurosci. Bull. 2020, 36, 91–95.
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59.
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9.
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411.
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell. Microbiol. 2006, 124, 783–801.
- Miller, S.I.; Ernst, R.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46.
- Werts, C.; Tapping, R.I.; Mathison, J.C.; Chuang, T.-H.; Kravchenko, V.V.; Girons, I.S.; Haake, D.A.; Godowski, P.J.; Hayashi, F.; Ozinsky, A.; et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2001, 2, 346–352.
- Que-Gewirth, N.L.; Ribeiro, A.A.; Kalb, S.R.; Cotter, R.J.; Bulach, D.M.; Adler, B.; Saint Girons, I.; Werts, C.; Raetz, C.R. A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A: The membrane anchor of an unusual lipopolysaccharide that activates TLR2. J. Biol. Chem. 2004, 279, 25420–25429.
- Nahori, M.-A.; Fournié-Amazouz, E.; Que-Gewirth, N.S.; Balloy, V.; Chignard, M.; Raetz, C.R.H.; Girons, I.S.; Werts, C. Differential TLR Recognition of Leptospiral Lipid A and Lipopolysaccharide in Murine and Human Cells. J. Immunol. 2005, 175, 6022–6031.
- Geijtenbeek, T.B.H.; Van Kooyk, Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS 2003, 111, 698–714.
- Urban, C.F.; Lourido, S.; Zychlinsky, A. How do microbes evade neutrophil killing? Cell. Microbiol. 2006, 8, 1687–1696.
- Schroder, K.; Tschopp, J. The inflammasomes. Cell Host Microbe 2010, 140, 821–832.
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582.
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000.
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891.
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32.
- Meri, T.; Murgia, R.; Stefanel, P.; Meri, S.; Cinco, M. Regulation of complement activation at the C3-level by serum resistant leptospires. Microb. Pathog. 2005, 39, 139–147.
- Barbosa, A.S.; Abreu, P.A.E.; Vasconcellos, S.A.; Morais, Z.M.; Gonçales, A.P.; Silva, A.S.; Daha, M.R.; Isaac, L. Immune Evasion of Leptospira Species by Acquisition of Human Complement Regulator C4BP. Infect. Immun. 2009, 77, 1137–1143.
- Gaudart, N.; Ekpo, P.; Pattanapanyasat, K.; Van Kooyk, Y.; Engering, A. Leptospira interrogans is recognized through DC-SIGN and induces maturation and cytokine production by human dendritic cells. FEMS Immunol. Med. Microbiol. 2008, 53, 359–367.
- Zhao, H.-Q.; Li, W.-M.; Lu, Z.-Q.; Sheng, Z.-Y.; Yao, Y.-M. The Growing Spectrum of Anti-Inflammatory Interleukins and Their Potential Roles in the Development of Sepsis. J. Interf. Cytokine Res. 2015, 35, 242–251.
- Ben, A.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296.
- Estavoyer, J.M.; Racadot, E.; Couetdic, G.; Leroy, J.; Grosperrin, L. Tumor Necrosis Factor in Patients with Leptospirosis. Rev. Infect. Dis. 1991, 13, 1245.
- Tajiki, M.H.; Salomão, R. Association of plasma levels of tumor necrosis factor α with severity of disease and mortality among patients with leptospirosis. Clin. Infect. Dis. 1996, 23, 1177–1178.
- Chirathaworn, C.; Supputtamongkol, Y.; Lertmaharit, S.; Poovorawan, Y. Cytokine levels as biomarkers for leptospirosis patients. Cytokine 2016, 85, 80–82.
- Wagenaar, J.F.P.; Gasem, M.H.; Goris, M.G.A.; Leeflang, M.; Hartskeerl, R.A.; van der Poll, T.; Veer, C.V.; van Gorp, E.C.M. Soluble ST2 Levels Are Associated with Bleeding in Patients with Severe Leptospirosis. PLoS Negl. Trop. Dis. 2009, 3, e453.
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172.
- Fialho, R.N.; Martins, L.; Pinheiro, J.P.; Bettencourt, B.F.; Couto, A.R.; Santos, M.R.; Peixoto, M.J.; Garrett, F.; Leal, J.; Tomás, A.M.; et al. Role of human leukocyte antigen, killer-cell immunoglobulin-like receptors, and cytokine gene polymorphisms in leptospirosis. Hum. Immunol. 2009, 70, 915–920.
- Matsui, M.; Roche, L.; Geroult, S.; Soupé-Gilbert, M.-E.; Monchy, D.; Huerre, M.; Goarant, C. Cytokine and Chemokine Expression in Kidneys during Chronic Leptospirosis in Reservoir and Susceptible Animal Models. PLoS ONE 2016, 11, e0156084.
- Bernardi, F.D.C.; Ctenas, B.; da Silva, L.F.F.; Nicodemo, A.C.; Saldiva, P.H.N.; Dolhnikoff, M.; Mauad, T. Immune receptors and adhesion molecules in human pulmonary leptospirosis. Hum. Pathol. 2012, 43, 1601–1610.
- Wagenaar, J.F.; Goris, M.G.; Gasem, M.H.; Isbandrio, B.; Moalli, F.; Mantovani, A.; Boer, K.R.; Hartskeerl, R.A.; Garlanda, C.; van Gorp, E.C. Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. J. Infect. 2009, 58, 425–432.
- Chin, V.; Lee, T.; Lim, W.; Ywy, W.S.; Syafinaz, A.; Zamberi, S.; Maha, A. Leptospirosis in human: Biomarkers in host immune responses. Microbiol. Res. 2018, 207, 108–115.
- Zhang, C.; Xu, J.; Zhang, T.; Qiu, H.; Li, Z.; Zhang, E.; Li, S.; Chang, Y.-F.; Guo, X.; Jiang, X.; et al. Genetic characteristics of pathogenic Leptospira in wild small animals and livestock in Jiangxi Province, China, 2002–2015. PLoS Negl. Trop. Dis. 2019, 13, e0007513.
- Esteves, L.; Bulhões, S.; Branco, C.C.; Mota, F.M.; Paiva, C.; Cabral, R.; Vieira, M.L.; Mota-Vieira, L. Human Leptospirosis: Seroreactivity and Genetic Susceptibility in the Population of São Miguel Island (Azores, Portugal). PLoS ONE 2014, 9, e108534.
- Cédola, M.; Chiani, Y.; Pretre, G.; Alberdi, L.; Vanasco, B.; Gómez, R.M. Association of Toll-like receptor 2 Arg753Gln and Toll-like receptor 1 Ile602Ser single-nucleotide polymorphisms with leptospirosis in an argentine population. Acta Trop. 2015, 146, 73–80.
- Charo, N.; Scharrig, E.; Ferrer, M.F.; Sanjuan, N.; Silva, E.A.C.; Schattner, M.; Gómez, R.M. Leptospira species promote a pro-inflammatory phenotype in human neutrophils. Cell. Microbiol. 2019, 21, e12990.
- Murgia, R.; Garcia, R.; Cinco, M. Leptospires Are Killed In Vitro by Both Oxygen-Dependent and -Independent Reactions. Infect. Immun. 2002, 70, 7172–7175.
- Vieira, M.L.; Teixeira, A.F.; Pidde, G.; Ching, A.T.C.; Tambourgi, D.V.; Nascimento, A.L.T.O.; Herwald, H. Leptospira interrogans outer membrane protein LipL21 is a potent inhibitor of neutrophil myeloperoxidase. Virulence 2018, 9, 414–425.
- Santecchia, I.; Ferrer, M.F.; Vieira, M.L.; Gómez, R.M.; Werts, C. Phagocyte Escape of Leptospira: The Role of TLRs and NLRs. Front. Immunol. 2020, 11, 571816.
- Scharrig, E.; Carestia, A.; Ferrer, M.F.; Cédola, M.; Pretre, G.; Drut, R.; Picardeau, M.; Schattner, M.; Gomez, R. Neutrophil Extracellular Traps are Involved in the Innate Immune Response to Infection with Leptospira. PLoS Negl. Trop. Dis. 2015, 9, e0003927.
- Zipfel, P.F.; Würzner, R.; Skerka, C. Complement evasion of pathogens: Common strategies are shared by diverse organisms. Mol. Immunol. 2007, 44, 3850–3857.
- Choy, H.A. Multiple Activities of LigB Potentiate Virulence of Leptospira interrogans: Inhibition of Alternative and Classical Pathways of Complement. PLoS ONE 2012, 7, e41566.
- Souza, N.M.; Vieira, M.L.; Alves, I.J.; de Morais, Z.M.; Vasconcellos, S.A.; Nascimento, A.L. Lsa30, a novel adhesin of Leptospira interrogans binds human plasminogen and the complement regulator C4bp. Microb. Pathog. 2012, 53, 125–134.
- Domingos, R.F.; Vieira, M.L.; Romero, E.C.; Gonçales, A.P.; de Morais, Z.M.; A Vasconcellos, S.; O Nascimento, A.L.T. Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions. BMC Microbiol. 2012, 12, 50.
- Barbosa, A.S.; Monaris, D.; Silva, L.B.; Morais, Z.M.; Vasconcellos, S.A.; Cianciarullo, A.M.; Isaac, L.; Abreu, P.A.E. Functional Characterization of LcpA, a Surface-Exposed Protein of Leptospira spp. That Binds the Human Complement Regulator C4BP. Infect. Immun. 2010, 78, 3207–3216.
This entry is offline, you can click here to edit this entry!
