Mucormycosis is a rare infection caused the members of the order Mucorales. Its prevalence ranges from 0.005 to 1.7 per million people worldwide, while in India, it reaches 14 cases per 100,000 inhabitants . During the COVID-19 pandemic, a surge in mucormycosis cases has been observed, especially in India, where the Government of India portal reported 47,508 cases from 5 May 2021 to 3 August 2021. Characteristically, Samir Joshi et al. reported 160 cases of COVID-19-associated mucormycosis (CAM) from April to May 2021 in the Ear, Nose, Throat Department of BJGMC-SGH hospital in India, compared with 3–8 cases of mucormycosis detected each year from 2016 to 2020.
- mucorales
- invasive fungal infections
- SARS-CoV-2
1. Clinical Presentation
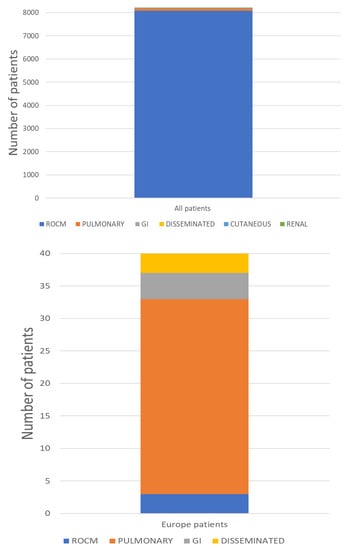
| Study | Incidence of CAM (%) | Type of Infection (%) |
Cerebral Involvement /ROCM pts, n (%) |
IMV or NIV n (%) |
DM (% of CAM pts) |
Steroids Intake (% of CAM pts) |
All-Cause Mortality (%) |
|---|---|---|---|---|---|---|---|
| Said Ahmed WM et al. [3] | NA | Maxillary osteomyelitis | 0/14 (0%) | NA | 64.2% DM 35.7% with temporary post-COVID-19 hyperglycemia |
NA | NA |
| Murthy R et al. [4] | NA | RO | 0/111 (0%) | NA | NA | NA | NA |
| Walia S et al. [5] | NA | SN (100%), O (51.85%), C (9.44%), Cu (1.85%), P (0.18%). | 51/529 (9.6%) | NA | 97.96% | 84.85% | 9.25% |
| Vare AA et al. [6] | 1.36% | ROCM | 3/67 (5%) | 18/67 (27%) | 90% | 84% | 34% |
| Fouad YA et al. [2] | NA | O | 0/26 (0%) | NA | 96.2% | 76.9% | 46,2% |
| Soni K et al. [7] | NA | ROCM | 29/145 (20%) | NA | 86.2% | 65% | 18% |
| Metwally MI et al. [8] | NA | Head and neck | 8/63 (12.7%) | NA | 80.9% | 82.5% | 17.5% |
| Arora U et al. [9] | NA | RS (29%), RO (47.3%), ROCM (14.5%), O (1.3%), RO/palatal (5.3%), Cu (0.6%), P (1.3%), D (0.6%) | 22/148 (14.9%) | NA | 92.1% | 65.8% | NA |
| Jindal G et al. [10] | NA | ROCM | 9/15 (60%) | NA | 100% | 80% | 6.6% |
| Syed-Abdul S et al. [11] | NA | NA | NA | NA | NA | NA | NA |
| Patel A et al. [12] | NA | RO (96.5%), P (3.4%) | 0/28 (0%) | NA | NA | NA | NA |
| Pruthi H et al. [13] | NA | P | NA | 0/5 (0%) | 100% | NA | 80% |
| Bansal SB et al. [14] | 10.8% | RO (91%), P (9%) | NA | 0/11 (0%) | 64%, 36% developed transient hyperglycemia |
100% | 18.2% |
| Dulski TM et al. [15] | NA | RO (10%), ROCM (30%), P (30%), D (20%), GI (10%) | 3/4 (75%) | 5/10 (50%) | 80% | 90% | 60% |
| Meshram HS et al. [16] | 4.4% | ROCM (91.8%), P (8.2%) | 11/42 (26.2%) | 0/61 (0%) | 24.6% | 44% | 26.2% |
| Aggarwal SK et al. [17] | NA | ROCM | 4/13 (30.8%) | NA | 92.3% | 92.3% | 15.4% |
| Kulkarni R et al. [18] | 2.1% (1 centre) | ROCM | 12/102 (11.8%) | NA | 81.6% | NA | 51%. |
| Choksi T et al. [19] | NA | ROCM | 6/73 (2%) | 17/73 (23.3%) | 74% | 98% | 36% |
| Kumar S et al. [20] | NA | ROCM | 60/287 (21%) | NA | 80% | NA | NA |
| Mehta R et al. [21] | NA | ROCM | 0/17 (0%) | NA | 100% | NA | NA |
| Panwar P et al. [22] | NA | ROCM | 0/7 (0%) | NA | 100% | 42.8% | 0% |
| Patel DD et al. [23] | NA | ROCM | 21/96 (21.9%) | 6/96 (6.3%) | 71.8% | 82.3% | NA |
| Vaid N et al. [24] | NA | ROCM | NA | NA | 33.8% | 100% | 10.7% |
| Goddanti N et al. [25] | NA | ROCM | NA | NA | 95.7% | 79% | NA |
| Yadav T et al. [26] | NA | ROCM | 25/50 (50%) | NA | 86% | 44% | NA |
| Meshram VB et al. [27] | NA | ROCM (90.9%), P (9%) | 3/10 (30%) | 0/11 (0%) | 54.5% | 100% | 27% |
| Zirpe K et al. [28] | NA | ROCM | 20/84 (23.8%) | NA | 64.3% | 83.3% | 15.5% |
| Alloush TK et al. [29] | NA | ROCM | 9/14 (64.2%) | 0/14 (0%) | 92.8% | NA | 21.4% |
| Pal P et al. [30] | NA | ROCM | 3/10 (30%) | ΝA | 70% | 80% | 30% |
| Danion F et al. [31] | NA | P(53%), GI (18%), ROCM (12%), D (18%) | NA | 13/17 (76.5%) | 47% | 76.5% | 88% |
| Nehara HR et al. [32] | NA | ROCM | 18/105 (17.1%) | NA | 78.1% | 66.3% | 19.05% |
| Pandiar D et al. [33] | NA | Oral | 0/12 (0%) | NA | 66,7% | NA | NA |
| Kumar S et al. [34] | NA | NA | NA | 0/55 (0%) | 83.6% | 98.2% | 16% |
| Bilgic A et al. [35] | 2.5% | ROCM | NA | 6/38 (16%) | 50% | 100% | 5% |
| Guemas E et al. [36] | 7.1% | P | NA | NA | 20% | 90% | 50% |
| Kumar SG et al. [37] | NA | ROCM | 44/101 (43.6%) | NA | 94% | 80.1% | 17.8% |
| Mani S et al. [38] | NA | ROCM | 4/89 (4.5%) | NA | 96% | 92% | 3.4% |
| Dravid A et al. [39] | NA | ROCM (98.3%), D (1.7%) | 26/58 (44.8%) | 3/59 (5.1%) | 89.8% | 100% | 25.4% |
| Naruka S et al. [40] | NA | ROCM | 9/79 (11.4%) | NA | 100% | NA | 18.18% |
| Jain K et al. [41] | NA | ROCM | 3/95 (3.2%) | NA | 77% | 100% | 5.2% |
| Bhanuprasad K et al. [42] | NA | ROCM | 39 (29.5%) | 3/132 (2.3%) | 97.7% | 55.3% | 9.8% |
| Desai EJ et al. [43] | NA | ROCM | 0/100 (0%) | NA | 80% | NA | 20% |
| Nasir\n et al. [44] | 0.35% | P (60%), ROCM (40%) | 4/4 (100%) | 3/10 (30%) | 70% | 80% | 70% |
| Gupta \s et al. [45] | NA | ROCM | 4/56 (7.1%) | NA | 85% | 66% | 16% |
| Joshi S et al. [46] | NA | ROCM | 22/178 (12.4%) | 5/178 (2.8%) | 74.2% | 52.8% | 15% |
| Pradhan P et al. [47] | NA | ROCM | 10/46 (21.7%) | NA | 95.65% | 89.1% | 19.5% |
| Mehta RNM et al. [48] | NA | ROCM | 33/215 (15.3%) | NA | 91% | 88% | 12.1% |
| Riad A et al. [49] | NA | ROCM | 7/7 (100%) | NA | 85.7% | 100% | 0% |
| Guzmán-Castro S et al. [50] | 0.04% | ROCM (83.3%), P(16.6%) | 5/5 (100%) | 2/6 (33.3%) | 83.3% | 100% | 83.3% |
| Seidel D et al. [51] | 2 centres: 0.67%, 0.58% ICU: 1.47%, 1.78% |
P(84.6%), ROCM (7.7%), GI (7.7%) | 1/1 (100%) | 11/13 (84.6%) | 23.07% | 84.6% | 53.8% |
| Gupta R et al. [52] | NA | ROCM | 25/115 (21.7%) | 13/115 (11.3%) | 85.2% | 100% | 21.7% |
| Alfishawy M et al. [53] | NA | ROCM (95.2%), P (4.8%) | 5/20 (25%) | NA | 90% | 100% | 33.3% |
| Dave TV et al. [54] | NA | ROCM | 19/58 (33%) | NA | 74% | NA | 34% |
| Selarka L et al. [55] | 1.8% | ROCM | 9/47 (19.1%) | 20/47 (42.6%) | 76.6% | 100% | 23.4% |
| Avatef Fazeli M et al. [56] | NA | ROCM | 0/12 (0%) | 1/12 (8.3%) | 83.33% | 75% | 66.7% |
| Mishra Y et al. [57] | 3.36% | ROCM | 0/32 (0%) | NA | 87.5% | 93% | 12.5% |
| Sen M et al. [58] | NA | ROCM | 539/2826 (19.1%) | 114/1602 (7.1) | 78% | 87% | 14% |
| Pakdel F et al. [59] | NA | ROCM | 7/15 (46%) | 1/15 (6.7%) | 86% | 46.6% | 47% |
| Y M. Reddy et al. [60] | NA | RO | 0/6 (0%) | NA | 100% | 66.7% | 100% |
| R. Arora et al. [61] | NA | ROCM | 6/60 (10%) | NA | 98.3% | 63.3% | NA |
| D.P Gupta et al. [62] | NA | ROCM | NA | NA | 100% | NA | 5.7% |
| M.Gautam et al. [63] | NA | ROCM | NA | NA | 100% | 66.7% | 0% |
| R.M.Mehta et al. [64] | NA | P | NA | 4/5 (80%) | 80% | 100% | 80% |
| Y.M.Reddy et al. [65] | NA | ROCM | NA | NA | 100% | 80.6% | 35.5% |
| S.P.Singh et al. [66] | NA | RO | 0/6 (0%) | 0/6 (0%) | 100% | 66.7% | 16.7% |
| M.Hada et al. [67] | NA | ROCM | 54/270 (20%) | NA | 92.2% | 72% | NA |
| M. Kumar H et al. [68] | NA | ROCM (85.7%), P (14.3%) | 15/24 (62.5%) | 6/28 (21.4%) | 75% | 70.4% | 73.9% |
| S. Bhandari et al. [69] | NA | NA | NA | NA | 86.8% | 84.3% | NA |
| M Chouhan et al. [70] | NA | ROCM | 9/41 (21.9%) | NA | 97.6% | 87.8% | 9.8% |
| Y. Singh et al. [71] | NA | ROCM (92.3%), P (7.7%) | 2/12 (16.7%) | 10/13 (76.9%) | 61.5% | 84.6% | 69.2% |
| S M Desai et al. [72] | NA | ROCM | 3/50 (6%) | NA | 82% | 84% | 30% |
| A. Kumari et al. [73] | NA | ROCM | 4/20 (20%) | NA | 80% | 80% | 30% |
| S. Mitra et al. [74] | NA | ROCM | NA | NA | 100% | 78.1% | NA |
| A Ramaswami et al. [75] | NA | ROCM | 17/70 (24.3%) | NA | 70% | 70% | NA |
| A.R. Joshi et al. [76] | NA | ROCM | 7/25 (28%) | 12/25 (48%) | 88% | 100% | 56% |
| A. Patel et al. [77] | 7 centers: 0.27% (general wards) | ROCM (86.1%), P (8.6%), renal (0.5%), other (e.g., Cu, GI) (2.7%), D (2.1%) | 44/161 (27.3%) | NA | 60.4% | 78.1% | 44.1% |
| S Sharma et al. [78] | NA | ROCM | 2/23 (8.7%) | NA | 91.3% | 100% | NA |
| R. Kant et al. [79] | NA | ROCM (96%), P (4%) | 11/96 (11.5%) | NA | 95% | 81% | 13% |
| C. Eker et al. [80] | NA | ROCM | 9/15 (60%) | NA | 100% | NA | 33.3% |
| A.K. Pandit et al. [81] | NA | ROCM | 30/56 (53.6%) | NA | 85.7% | 53.6% | 30.6% |
| S.F. Youssif et al. [82] | 7.6% | ROCM | 32/33 (97%) | NA | 63.6% | NA | 90.9% |
| A. Sekaran, et al. [83] | NA | ROCM | 6/30 (20%) | 8/30 (26.7%) | 100% | 90% | 16.7% |
| R. R. Shabana et al. [84] | NA | ROCM | 4/30 (13.3%) | 1/30 (3.3%) | 90% | 66.6% | 20% |
| A. K Patel et al. [85] | NA | ROCM (92.2%), P (7.8%) | 5/59 (8.5%) | NA | 75% | 90.6% | 4.7% |
| H. D.D. Martins et al. [86] | NA | ROCM | 0/6 (0%) | NA | 83.3% | NA | 16.7% |
| S. Iqtadar et al. [87] | NA | ROCM | NA | 0/7 (0%) | 71.4% | 100% | 14.3% |
| A. Al Balushi et al. [88] | NA | ROCM | 3/10 (30%) | 6/10 (60%) | 100% | 30% | 60% |
| R. Soman et al. [89] | NA | ROCM (78.6%), P (21.4%) | 5/22 (22.7%) | NA | NA | NA | 25% |
2. Diagnosis
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12123092
References
- Leal-Buitrago, A.; Mondragon-Ángulo, D.; Cely-Aldana, N.A.; Ortega-Sierra, M.G.; Bolaño-Romero, M.P. Letter: Spectrum of hospitalized NeuroCOVID diagnoses from a tertiary care neurology centre in Eastern India. J. Clin. Neurosci. 2022, 96, 227–228.
- Fouad, Y.A.; Bakre, H.M.; Nassar, M.A.; Gad, M.O.A.; Shaat, A.A.K. Characteristics and Outcomes of a Series of COVID-Associated Mucormycosis Patients in Two Different Settings in Egypt Through the Third Pandemic Wave. Clin. Ophthalmol. 2021, 15, 4795–4800.
- Ahmed, W.M.S.; Elsherbini, A.M.; Elsherbiny, N.M.; El-Sherbiny, M.; Ramzy, N.I.; Arafa, F.A. Maxillary Mucormycosis Osteomyelitis in Post COVID-19 Patients: A Series of Fourteen Cases. Diagnostics 2021, 11, 2050.
- Murthy, R.; Bagchi, A.; Gote, Y.S. Role of medial orbital wall decompression in COVID-19-associated rhino-orbital mucormycosis management. Indian J. Ophthalmol. 2021, 69, 3795–3796.
- Walia, S.; Bhaisare, V.; Rawat, P.; Kori, N.; Sharma, M.; Gupta, N.; Urdhwareshwar, S.; Thakur, S.; Arya, N. COVID-19-associated mucormycosis: Preliminary report from a tertiary eye care centre. Indian J. Ophthalmol. 2021, 69, 3685–3689.
- Vare, A.A.; Yellambkar, S.; Farheen, A.; Nandedkar, V.; Bhombe, S.S.; Shah, R. Incidence, cumulative mortality and factors affecting the outcome of COVID-19-associated mucormycosis from Western India. Indian J. Ophthalmol. 2021, 69, 3678–3683.
- Soni, K.; Das, A.; Sharma, V.; Goyal, A.; Choudhury, B.; Chugh, A.; Kumar, D.; Yadav, T.; Jain, V.; Agarwal, A.; et al. Sanje Surgical & medical management of ROCM (Rhino-orbito-cerebral mucormycosis) epidemic in COVID-19 era and its outcomes—A tertiary care center experience. J. Mycol. Med. 2021, 32, 101238.
- Metwally, M.I.; Mobashir, M.; Sweed, A.H.; Mahmoud, S.M.; Hassan, A.G.; ElKashishy, K.; Eesa, M.; Elnashar, I.; Elmalt, A.; Elsayed, A.I.; et al. Post COVID-19 Head and Neck Mucormycosis: MR Imaging Spectrum and Staging. Acad. Radiol. 2021, 29, 674–684.
- Arora, U.; Priyadarshi, M.; Katiyar, V.; Soneja, M.; Garg, P.; Gupta, I.; Bharadiya, V.; Berry, P.; Ghosh, T.; Patel, L.; et al. Risk factors for Coronavirus disease-associated mucormycosis. J. Infect. 2021, 84, 383–390.
- Jindal, G.; Sethi, A.; Bhargarva, K.; Sethi, S.; Mittal, A.; Singh, U.; Singh, S.; Shrivastava, A. Imaging findings in invasive rhino-orbito-cerebral mucormycosis in post-COVID-19 patients. Bayl. Univ. Med. Cent. Proc. 2021, 351, 32–34.
- Syed-Abdul, S.; Babu, A.S.; Bellamkonda, R.S.; Itumalla, R.; Acharyulu, G.; Krishnamurthy, S.; Ramana, Y.V.S.; Mogilicharla, N.; Malwade, S.; Li, Y.-C. Using artificial intelligence-based models to predict the risk of mucormycosis among COVID-19 survivors: An experience from a public hospital in India. J. Infect. 2021, 84, 351–354.
- Patel, A.; Patel, K.; Patel, K.; Shah, K.; Chakrabarti, A. Therapeutic drug monitoring of posaconazole delayed release tablet while managing COVID-19-associated mucormycosis in a real-life setting. Mycoses 2021, 65, 312–316.
- Pruthi, H.; Muthu, V.; Bhujade, H.; Sharma, A.; Baloji, A.; Ratnakara, R.G.; Bal, A.; Singh, H.; Sandhu, M.S.; Negi, S.; et al. Pulmonary Artery Pseudoaneurysm in COVID-19-Associated Pulmonary Mucormycosis: Case Series and Systematic Review of the Literature. Mycopathologia 2021, 187, 31–37.
- Bansal, S.B.; Rana, A.; Babras, M.; Yadav, D.; Jha, P.; Jain, M.; Sethi, S.K. Risk factors and outcomes of COVID associated mucormycosis in kidney transplant recipients. Transpl. Infect. Dis. 2021, 24, e13777.
- Dulski, T.M.; DeLong, M.; Garner, K.; Patil, N.; Cima, M.J.; Rothfeldt, L.; Gulley, T.; Porter, A.; Vyas, K.S.; Liverett, H.K.; et al. Notes from the Field: COVID-19-Associated Mucormycosis—Arkansas, July–September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1750–1751.
- Meshram, H.S.; Kute, V.B.; Yadav, D.K.; Godara, S.; Dalal, S.; Guleria, S.; Bhalla, A.K.; Pathak, V.; Anandh, U.; Bansal, S.; et al. Impact of COVID-19-associated Mucormycosis in Kidney Transplant Recipients: A Multicenter Cohort Study. Transplant. Direct. 2021, 8, e1255.
- Aggarwal, S.K.; Kaur, U.; Talda, D.; Pandey, A.; Jaiswal, S.; Kanakan, A.; Singh, A.; Chakrabarti, S.S. Case Report: Rhino-orbital Mucormycosis Related to COVID-19: A Case Series Exploring Risk Factors. Am. J. Trop. Med. Hyg. 2022, 106, 566–570.
- Kulkarni, R.; Pujari, S.S.; Gupta, D.; Ojha, P.; Dhamne, M.; Bolegave, V.; Dhonde, P.; Soni, A.; Adwani, S.; Diwan, A.; et al. Cerebrovascular Involvement in Mucormycosis in COVID-19 Pandemic. J. Stroke Cerebrovasc. Dis. 2021, 31, 106231.
- Choksi, T.; Agrawal, A.; Date, P.; Rathod, D.; Gharat, A.; Ingole, A.; Chaudhari, B.; Pawar, N. Cumulative Mortality and Factors Associated with Outcomes of Mucormycosis after COVID-19 at a Multispecialty Tertiary Care Center in India. JAMA Ophthalmol. 2022, 140, 66–72.
- Kumar, S.; Choudhary, R.; Pandey, V.P. “MuCovid-21” study: Mucormycosis at an Indian tertiary care centre during the COVID-19 pandemic. J. R. Coll. Physicians Edinb. 2021, 51, 352–358.
- Mehta, R.; Nagarkar, N.M.; Ksbs, K.S.; Ty, S.S.; Arora, R.D.; Aggarwal, A. Facial Nerve Palsy in COVID-19-Associated Mucormycosis Patients: A Case Series. Cureus 2021, 13, e19208.
- Panwar, P.; Gupta, A.; Kumar, A.; Gupta, B.; Navriya, S.C. Mucormycosis in COVID Diabetic Patients: A Horrifying Triad! Indian J. Crit. Care Med. 2021, 25, 1314–1317.
- Patel, D.D.; Adke, S.; Badhe, P.V.; Lamture, S.; Marfatia, H.; Mhatre, P. COVID-19 associated Rhino-Orbito-Cerebral Mucormycosis: Imaging spectrum and Clinico-radiological correlation—A single Centre experience. Clin. Imaging 2021, 82, 172–178.
- Vaid, N.; Mishra, P.; Gokhale, N.; Vaid, S.; Vaze, V.; Kothadiya, A.; Deka, T.; Agarwal, R. A Proposed Grading System and Experience of COVID-19 Associated Rhino Orbito Cerebral Mucormycosis from an Indian Tertiary Care Cente. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3505–3512.
- Goddanti, N.; Reddy, Y.M.; Kumar, M.K.; Rajesh, M.; Reddy, L.S. Role of COVID 19 Inflammatory Markers in Rhino-Orbito-Cerebral Mucormycosis: A Case Study in Predisposed Patients at a Designated Nodal Centre. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3498–3504.
- Yadav, T.; Tiwari, S.; Gupta, A.; Garg, P.K.; Khera, P.S.; Rajagopal, R.; Goyal, A.; Soni, K.; Chugh, A.; Jain, V.; et al. Magnetic Resonance Imaging in Coronavirus Disease—2019 Associated Rhino-Orbital-Cerebral Mucormycosis (CA-ROCM)—Imaging Analysis of 50 Consecutive Patients. Curr. Probl. Diagn. Radiol. 2022, 51, 112–120.
- Meshram, H.S.; Kute, V.B.; Chauhan, S.; Dave, R.; Patel, H.; Banerjee, S.; Desai, S.; Kumar, D.; Navadiya, V.; Mishra, V. Mucormycosis as SARS-CoV2 sequelae in kidney transplant recipients: A single-center experience from India. Int. Urol. Nephrol. 2021, 54, 1693–1703.
- Zirpe, K.; Pote, P.; Deshmukh, A.; Gurav, S.K.; Tiwari, A.M.; Suryawanshi, P. A Retrospective Analysis of Risk Factors of COVID-19 Associated Mucormycosis and Mortality Predictors: A Single-Center Study. Cureus 2021, 13, e18718.
- Alloush, T.K.; Mansour, O.; Alloush, A.T.; Roushd, T.; Hamid, E.; El-Shamy, M.; Shokri, H.M. Rhino-orbito-cerebral mucormycosis during the COVID-19 third wave in 2021: An Egyptian preliminary report from a single tertiary hospital. Neurol. Sci. 2021, 43, 799–809.
- Pal, P.; Chatterjee, N.; Ghosh, S.; Ray, B.K.; Mukhopadhyay, P.; Bhunia, K.; Srivastava, S.R.; Adhikari, S.; Barman, D.; Banerjee, B.; et al. COVID Associated Mucormycosis: A Study on the Spectrum of Clinical, Biochemical and Radiological Findings in A Series of Ten Patients. J. Assoc. Physicians India 2021, 69, 11–12.
- Danion, F.; Letscher-Bru, V.; Guitard, J.; Sitbon, K.; Dellière, S.; Angoulvant, A.; Desoubeaux, G.; Botterel, F.; Bellanger, A.-P.; Gargala, G.; et al. Coronavirus Disease 2019-Associated Mucormycosis in France: A Rare but Deadly Complication. Open Forum Infect. Dis. 2021, 9, ofab566.
- Nehara, H.R.; Kumawat, S.; Gupta, J.; Gupta, G.; Sirohi, P.; Ih, S.; Gupta, B. Coronavirus Disease, Diabetes and Glucocorticoid a Terrible Trio for Invasive Mucormycosis: An Observational Study from Northwest Rajasthan. J. Assoc. Physicians India 2022, 69, 11–12.
- Pandiar, D.; Ramani, P.; Krishnan, R.P.; Dinesh, Y. Histopathological analysis of soft tissue changes in gingival biopsied specimen from patients with underlying corona virus disease associated mucormycosis (CAM). Med. Oral Patol. Oral Cir. Bucal. 2022, 27, e216–e222.
- Kumar, S.; Acharya, S.; Jain, S.; Shukla, S.; Talwar, D.; Shah, D.; Hulkoti, V.; Parveen, S.; Patel, M.; Patel, S. Role of Zinc and Clinicopathological Factors for COVID-19-Associated Mucormycosis (CAM) in a Rural Hospital of Central India: A Case-Control Study. Cureus 2022, 14, e22528.
- Bilgic, A.; Kodjikian, L.; Sudhalkar, A.; Dwivedi, S.; Vasavada, V.; Shah, A.; Dziadzko, M.; Mathis, T. Risk Factors for COVID-19 Associated Mucormycosis: The Ophthalmologist’s Perspective. J. Fungi 2022, 8, 271.
- Guemas, E.; Cassaing, S.; Malavaud, S.; Fillaux, J.; Chauvin, P.; Lelièvre, L.; Ruiz, S.; Riu, B.; Berry, A.; Iriart, X. A Clustered Case Series of Mucorales Detection in Respiratory Samples from COVID-19 Patients in Intensive Care, France, August to September 2021. J. Fungi 2022, 8, 258.
- Gg, S.K.; Deepalam, S.; Siddiqui, A.; Adiga, C.P.; Kumar, S.; Shivalingappa, S.S.; Acharya, U.V.; Goolahally, L.N.; Sharma, S.; Andrew, D.; et al. Coronavirus Disease 2019 (COVID-19)-Associated Rhino-Orbito-Cerebral Mucormycosis: A Multi-Institutional Retrospective Study of Imaging Patterns. World Neurosurg. 2022, 162, e131–e140.
- Mani, S.; Thirunavukkarasu, A. A clinico-pathological study of COVID-19 associated rhino-orbital-cerebral mucormycosis. Indian J. Ophthalmol. 2022, 70, 1013–1018.
- Dravid, A.; Kashiva, R.; Khan, Z.; Bande, B.; Memon, D.; Kodre, A.; Mane, M.; Pawar, V.; Patil, D.; Kalyani, S.; et al. Epidemiology, clinical presentation and management of COVID-19 associated mucormycosis: A single centre experience from Pune, Western India. Mycoses 2022, 65, 526–540.
- Naruka, S.; Rana, N.; Singh, N.; Kishore, A.; Nagpal, K. COVID-19 associated rhino-orbital-cerebral mucormycosis-an institutional series. Ear Nose Throat J. 2022.
- Jain, K.; Surana, A.; Choudhary, T.S.; Vaidya, S.; Nandedkar, S.; Purohit, M. Clinical and histology features as predictor of severity of mucormycosis in post-COVID-19 patients: An experience from a rural tertiary setting in Central India. SAGE Open Med. 2022, 10.
- Bhanuprasad, K.; Manesh, A.; Devasagayam, E.; Varghese, L.; Cherian, L.M.; Kurien, R.; Karthik, R.; Deodhar, D.; Vanjare, H.; Peter, J.; et al. Risk factors associated with the mucormycosis epidemic during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 111, 267–270.
- Desai, E.J.; Pandya, A.; Upadhya, I.; Patel, T.; Banerjee, S.; Jain, V. Epidemiology, Clinical Features and Management of Rhino Orbital Mucormycosis in Post COVID 19 Patients. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 103–107.
- Nasir, N.; Farooqi, J.; Mahmood, S.F.; Jabeen, K. COVID-19 associated mucormycosis: A life-threatening complication in patients admitted with severe to critical COVID-19 from Pakistan. Clin. Microbiol. Infect. 2021, 27, 1704–1707.
- Gupta, S.; Ahuja, P. Risk Factors for Procurence of Mucormycosis and its Manifestations Post Covid-19: A Single Arm Retrospective Unicentric Clinical Study. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3131–3138.
- Joshi, S.; Telang, R.; Tambe, M.; Havaldar, R.; Sane, M.; Shaikh, A.; Roy, C.; Yathati, K.; Sonawale, S.; Borkar, R.; et al. Outbreak of Mucormycosis in Coronavirus Disease Patients, Pune, India. Emerg. Infect. Dis. 2022, 28, 1–8.
- Pradhan, P.; Shaikh, Z.; Mishra, A.; Preetam, C.; Parida, P.K.; Sarkar, S.; Samal, D.K.; Nayak, A.; Chadaram, S.; Das, K.K.; et al. Predisposing factors of rhino-orbital-cerebral mucormycosis in patients with COVID 19 infection. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3151–3157.
- Mehta, R.; Nagarkar, N.M.; Jindal, A.; Rao, K.N.; Nidhin, S.B.; Arora, R.D.; Sharma, A.; Wankhede, A.; Satpute, S.; Chakravarty, S.; et al. Multidisciplinary Management of COVID-Associated Mucormycosis Syndemic in India. Indian J. Surg. 2021, 84, 934–942.
- Riad, A.; Shabaan, A.A.; Issa, J.; Ibrahim, S.; Amer, H.; Mansy, Y.; Kassem, I.; Kassem, A.B.; Howaldt, H.; Klugar, M.; et al. COVID-19-Associated Mucormycosis (CAM): Case-Series and Global Analysis of Mortality Risk Factors. J. Fungi 2021, 7, 837.
- Guzmán-Castro, S.; Chora-Hernandez, L.D.; Trujillo-Alonso, G.; Calvo-Villalobos, I.; Sanchez-Rangel, A.; Ferrer-Alpuin, E.; Ruiz-Jimenez, M.; Corzo-Leon, D.E. COVID-19-associated mucormycosis, diabetes and steroid therapy: Experience in a single centre in Western Mexico. Mycoses 2021, 65, 65–70.
- Seidel, D.; Simon, M.; Sprute, R.; Lubnow, M.; Evert, K.; Speer, C.; Seeßle, J.; Khatamzas, E.; Merle, U.; Behrens, C.; et al. Results from a national survey on COVID-19-associated mucormycosis in Germany: 13 patients from six tertiary hospitals. Mycoses 2021, 65, 103–109.
- Gupta, R.; Kesavadev, J.; Krishnan, G.; Agarwal, S.; Saboo, B.; Shah, M.; Mittal, A.; Durani, S.; Luthra, A.; Singhal, A.; et al. COVID-19 associated mucormycosis: A Descriptive Multisite Study from India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102322.
- Alfishawy, M.; Elbendary, A.; Younes, A.; Negm, A.; Hassan, W.S.; Osman, S.H.; Nassar, M.; Elanany, M.G. Diabetes mellitus and Coronavirus Disease (Covid-19) Associated Mucormycosis (CAM): A wake-up call from Egypt. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102195.
- Dave, T.V.; Nair, A.G.; Hegde, R.; Vithalani, N.; Desai, S.; Adulkar, N.; Kamal, S.; Mittal, R.; Bradoo, R.A. Clinical Presentations, Management and Outcomes of Rhino-Orbital-Cerebral Mucormycosis (ROCM) Following COVID-19: A Multi-Centric Study. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 488–495.
- Selarka, L.; Sharma, S.; Saini, D.; Sharma, S.; Batra, A.; Waghmare, V.T.; Dileep, P.; Patel, S.; Shah, M.; Parikh, T.; et al. Mucormycosis and COVID-19: An epidemic within a pandemic in India. Mycoses 2021, 64, 1253–1260.
- Fazeli, M.A.; Rezaei, L.; Javadirad, E.; Iranfar, K.; Khosravi, A.; Saman, J.A.; Poursabbagh, P.; Ghadami, M.R.; Parandin, M.M.; Dehghani, A.; et al. Increased incidence of rhino-orbital mucormycosis in an educational therapeutic hospital during the COVID-19 pandemic in western Iran: An observational study. Mycoses 2021, 64, 1366–1377.
- Mishra, Y.; Prashar, M.; Sharma, D.; Akash; Kumar, V.P.; Tilak, T. Diabetes, COVID 19 and mucormycosis: Clinical spectrum and outcome in a tertiary care medical center in Western India. Diabetes Metab. Syndr. 2021, 15, 102196.
- Honavar, S.; Sen, M.; Sengupta, S.; Rao, R.; Kim, U.; Sharma, M.; Sachdev, M.; Grover, A.; Surve, A.; Budharapu, A.; et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India—Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021, 69, 1670–1692.
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Rezaie, Y.; Rajaeih, S.; Alijani, N.; Barac, A.; et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses 2021, 64, 1238–1252.
- Reddy, Y.M.; Parida, S.; Reddy, S.B.; Yeduguri, S.; Pidaparthi, L.; Jaiswal, S.K.; Sadhvani, B.; Murthy, J.M.K. Decoding “guitar pick sign” in COVID-19-associated mucormycosis: A case series. Indian J. Ophthalmol. 2022, 70, 1425–1427.
- Arora, R.; Goel, R.; Khanam, S.; Kumar, S.; Shah, S.; Singh, S.; Chhabra, M.; Meher, R.; Khurana, N.; Sagar, T.; et al. Rhino-Orbito-Cerebral-Mucormycosis During the COVID-19 Second Wave in 2021—A Preliminary Report from a Single Hospital. Clin. Ophthalmol. 2021, 15, 3505–3514.
- Gupta, D.P.; Gupta, S.; Shah, C.K.; Sreevidya, S.R. Clinical Study of Surge of Mucormycosis in COVID-19 Pandemic: A Tertiary Care Center Study. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3455–3462.
- Gautam, M.; Soni, M.; Bhaisare, V.; Rawat, P.; Walia, S.; Kori, N. Complete and incomplete lower motor neuron facial palsy in post-COVID-19 mucormycosis. Indian J. Ophthalmol. 2022, 70, 1365–1370.
- Mehta, R.M.; Bansal, S.; Kalpakkam, H. Critical COVID-19-associated pulmonary mucormycosis: The underreported life-threatening spectrum of the mucormycosis epidemic. Lung India 2022, 39, 187–190.
- Reddy, Y.M.; Yeduguri, S.; Reddy, N.V.S.; Parida, S.; Kamatham, S.N.; Pidaparthi, L.; Jaiswal, S.K.; Sadhvani, B.; Tourani, V.; Kumar, S.; et al. Pathogenetic factors fanning the flames of COVID-19 to cause rhino-orbito-cerebral mucormycosis: An observational study. J. Med. Mycol. 2022, 32, 101252.
- Singh, S.P.; Rana, J.; Singh, V.K.; Singh, R.; Sachan, R.; Singh, S.; Jain, S. Rhino-orbital mucormycosis: Our experiences with clinical features and management in a tertiary care center. Rom. J. Ophthalmol. 2021, 65, 339–353.
- Hada, M.; Gupta, P.; Bagarhatta, M.; Tripathy, K.; Harsh, A.; Khilnani, K.; Mendiratta, K.; Agarwal, S.; Chouhan, J.K.; Bhandari, S. Orbital magnetic resonance imaging profile and clinicoradiological correlation in COVID-19-associated rhino-orbital-cerebral mucormycosis: A single-center study of 270 patients from North India. Indian J. Ophthalmol. 2022, 70, 641–648.
- Kumar, H.M.; Sharma, P.; Rudramurthy, S.M.; Sehgal, I.S.; Prasad, K.T.; Pannu, A.K.; Das, R.; Panda, N.K.; Sharma, N.; Chakrabarti, A.; et al. Serum iron indices in COVID-19-associated mucormycosis: A case-control study. Mycoses 2021, 65, 120–127.
- Bhandari, S.; Bhargava, S.; Samdhani, S.; Singh, S.N.; Sharma, B.B.; Agarwal, S.; Sharma, M.P.; Sharma, S.; Sharma, V.; Kakkar, S.; et al. COVID-19, Diabetes and Steroids: The Demonic Trident for Mucormycosis. Indian J. Otolaryngol. Head Neck Surg. 2021, 74, 3469–3472.
- Chouhan, M.; Solanki, B.; Shakrawal, N. Rhino-orbital-cerebral mucormycosis: Fungal epidemic in a viral pandemic. J. Laryngol. Otol. 2021, 135, 981–986.
- Singh, Y.; Ganesh, V.; Kumar, S.; Patel, N.; Aggarwala, R.; Soni, K.D.; Trikha, A. Coronavirus Disease-Associated Mucormycosis from a Tertiary Care Hospital in India: A Case Series. Cureus 2021, 13, e16152.
- Desai, S.M.; Gujarathi-Saraf, A.; Agarwal, E.A. Imaging findings using a combined MRI/CT protocol to identify the “entire iceberg” in post-COVID-19 mucormycosis presenting clinically as only “the tip”. Clin. Radiol. 2021, 76, 784.e27–784.e33.
- Kumari, A.; Rao, N.P.; Patnaik, U.; Malik, V.; Tevatia, M.S.; Thakur, S.; Jaydevan, J.; Saxena, P. Management outcomes of mucormycosis in COVID-19 patients: A preliminary report from a tertiary care hospital. Med. J. Armed Forces India 2021, 77 (Suppl. 2), S289–S295.
- Mitra, S.; Janweja, M.; Sengupta, A. Post-COVID-19 rhino-orbito-cerebral mucormycosis: A new addition to challenges in pandemic control. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 2417–2422.
- Ramaswami, A.; Sahu, A.K.; Kumar, A.; Suresh, S.; Nair, A.; Gupta, D.; Chouhan, R.; Bhat, R.; Mathew, R.; Majeed, J.A.; et al. COVID-19-associated mucormycosis presenting to the Emergency Department-an observational study of 70 patients. Qjm Int. J. Med. 2021, 114, 464–470.
- Joshi, A.R.; Muthe, M.M.; Patankar, S.H.; Athawale, A.; Achhapalia, Y. CT and MRI Findings of Invasive Mucormycosis in the Setting of COVID-19: Experience from a Single Center in India. Am. J. Roentgenol. 2021, 217, 1431–1432.
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease-Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359.
- Sharma, S.; Grover, M.; Bhargava, S.; Samdani, S.; Kataria, T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021, 135, 442–447.
- Kant, R.; Totaganti, M.; Mohan, B.; Bairwa, M.; Panda, P.K.; Tyagi, A.; Prasad, A.; Bahurupi, Y. Clinical Characteristics of 100 Patients With COVID-19-Associated Mucormycosis From a Tertiary Care Center in North India. Cureus 2022, 14, e25652.
- Eker, C.; Tarkan, O.; Surmelioglu, O.; Dagkiran, M.; Tanrisever, I.; Karakaya, S.P.Y.; Ulas, B.; Onan, E.; Uguz, A.H.; Ozdemir, S. Alternating pattern of rhino-orbital-cerebral mucormycosis with COVID-19 in diabetic patients. Eur. Arch. Otorhinolaryngol. 2022.
- Pandit, A.K.; Tangri, P.; Misra, S.; Srivastava, M.V.P.; Bhatnagar, S.; Thakar, A.; Sikka, K.; Panda, S.; Vishnu, V.Y.; Singh, R.K.; et al. Mucormycosis in COVID-19 Patients: A Case-Control Study. Microorganisms 2022, 10, 1209.
- Youssif, S.F.; Abdelrady, M.M.; Thabet, A.A.; Abdelhamed, M.A.; Gad, M.O.A.; Abu-Elfatth, A.M.; Saied, G.M.; Goda, I.; Algammal, A.M.; Batiha, G.E.-S.; et al. COVID-19 associated mucormycosis in Assiut University Hospitals: A multidisciplinary dilemma. Sci. Rep. 2022, 12, 10494.
- Sekaran, A.; Patil, N.; Sabhapandit, S.; Sistla, S.K.; Reddy, D.N. Rhino-orbito-cerebral mucormycosis: An epidemic in a pandemic. IJID Reg. 2021, 2, 99–106.
- Shabana, R.R.; Eldesouky, M.A.; Elbedewy, H.A. Exenterate or Not: A Simple Proposed Management Algorithm for Mucormycosis During the Era of COVID-19 in a Tertiary Eye Care Center in Egypt. Clin. Ophthalmol. 2022, 16, 1933–1940.
- Patel, A.K.; Bakshi, H.; Shah, K.; Patel, S.; Patel, T.; Patel, K.; Patel, K.K. Risk factors for COVID-19 associated mucormycosis in India: A case control study. Med. Mycol. 2022, 60, myac044.
- Martins, H.D.; Pares, A.R.; Martínez, A.T.; Guevara, R.A.P.; Inaoka, S.D.; Costa, D.F.; Leal, C.B.; Soares, C.D.; da Paz, A.R.; Perez, D.E.d.C.; et al. A case series of mucormycosis after covid infection in two hospitals. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e757–e759.
- Iqtadar, S.; Hashmat, M.; Chaudhry, M.N.A.; Mumtaz, S.U.; Abaidullah, S.; Pascual-Figal, D.A.; Khan, A. Unnecessary Use of Corticosteroids for managing early mild symptoms of COVID-19 may lead to Rhino-ortibal-cerebral mucormycosis in Patients with Diabetes—A case series from Lahore, Pakistan. Ther. Adv. Infect. Dis. 2022, 9.
- Al Balushi, A.; Al Ajmi, A.; Al Sinani, Q.; Menon, V.; Al Berieki, Z.; Al Shezawi, A.; Al Azri, S.; Al Rashdi, A.; Al Jardani, A.; Al Baluki, T.; et al. COVID-19-Associated Mucormycosis: An Opportunistic Fungal Infection. A Case Series and Review. Int. J. Infect. Dis. 2022, 121, 203–210.
- Soman, R.; Chakraborty, S.; Joe, G. Posaconazole or isavuconazole as sole or predominant antifungal therapy for COVID-19-associated mucormycosis. A retrospective observational case series. Int. J. Infect. Dis. 2022, 120, 177–178.
- Pai, V.; Sansi, R.; Kharche, R.; Bandili, S.C.; Pai, B. Rhino-orbito-cerebral Mucormycosis: Pictorial Review. Insights Imaging 2021, 12, 167.
- Krishna, V.; Bansal, N.; Morjaria, J.; Kaul, S. COVID-19-Associated Pulmonary Mucormycosis. J. Fungi 2022, 8, 11.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421.
- Samson, R.; Dharne, M. COVID-19 associated mucormycosis: Evolving technologies for early and rapid diagnosis. 3 Biotech. 2021, 12, 6.
- Fathima, A.S.; Mounika, V.L.; Kumar, V.U.; Gupta, A.K.; Garapati, P.; Ravichandiran; Dhingra, S.; Murtia, K. Mucormycosis: A triple burden in patients with diabetes during COVID-19 Pandemic. Health Sci. Rev. 2021, 1, 100005.
- Gupta, P.; Malhotra, H.S.; Saxena, P.; Singh, R.; Shukla, D.; Hasan, M.S.; Verma, V.; Banerjee, G.; Puri, B.; Dandu, H. Utility of itraconazole and terbinafine in mucormycosis: A proof-of-concept analysis. J. Investig. Med. 2022, 70, 914–918.
- Ganesan, N.; Sivanandam, S. Histomorphological features of mucormycosis with rise and fall of COVID-19 pandemic. Pathol. Res. Pract. 2022, 236.
- Dimopoulos, G.; Almyroudi, M.-P.; Myrianthefs, P.; Rello, J. COVID-19-Associated Pulmonary Aspergillosis (CAPA). J. Intensive Med. 2021, 1, 71–80.
