Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hydrothermal liquefaction (HTL) is a thermochemical depolymerization technology, also known as hydrous pyrolysis, that transforms wet biomass into biocrude and valuable chemicals at a moderate temperature (usually 200–400 °C) and high pressure (typically 10–25 MPa). In HTL, water acts as a key reactant in HTL activities. The reaction’s process parameters mostly influence the distribution, composition, and characteristics of HTL products; therefore, optimizing those factors can aid in producing biocrude with a high yield and suitable quality.
- HTL
- lignocellulosic biomass
- protein-containing biomass
- biocrude
1. Types of Lignocellulose
The yield and makeup of biocrude, the primary product, are highly correlated with those of lignocellulose [1]. In Figure 1, a few typical biomasses used for HTL are depicted. This is mostly because lignin has a more stable structure than other components, making it less likely to decay and liquefy to form biocrude. In contrast, cellulose and hemicellulose have simple structures, are less stable, and are, therefore, more likely to degrade and liquefy. Feng et al. [2] treated white birch, white spruce, and white pine bark with ethanol-water (50:50 v/v) at 300 °C for 15 min. The findings demonstrated that feedstocks with lower lignin concentration and higher ash content could boost conversion rates and biocrude yields. Liquidized white birch bark had the highest biocrude yield (67%), followed by white spruce bark (58%) and white pine bark’s lowest (36%) levels. Due to lignin’s higher thermal stability, its breakdown requires a higher temperature than cellulose and hemicellulose. Lignin is thermally more stable than the other biomass, and the order of hydrothermal conversion degree of biomass and biomass components was as follows: cellulose, sawdust, rice husk, and then lignin [3]. In the liquefaction of switchgrass in subcritical water, the residue solid mainly contained lignin fractions [4][5]. Softwood biomass contains higher lignin content than hardwood biomass. It was reported that the lignin-rich cypress (softwood biomass) produced hydrocarbons with a major portion of phenolic hydrocarbons and derivatives than cherry (hardwood biomass), while the formation of acetic acid was more in the hemicellulose and cellulose-rich cherry than cypress [6]. In the hydrothermal conversion of the mixtures from different ratios of cellulose to lignin, the char yields increased with the increasing lignin content, and the yields of gas and aqueous soluble products increased with the increasing cellulose content, but it was difficult to conclude the biocrude yields change with increasing lignin content [7]. Belkheir et al. In this study, softwood Kraft lignin was depolymerized using subcritical water (623 K; 25 MPa) in a continuous, small pilot unit with a flow rate of 2 kg/h. ZrO2, K2CO3/KOH, and Na2CO3/NaOH were used as catalytic systems and phenol as the capping agent. The influence of the ratio between sodium and potassium in the feed on the yield and composition of the product stream was investigated. The results showed that biocrude, water-soluble organics (WSO), and char yields were not remarkably influenced by shifting the catalytic system from potassium to sodium [8]. Yang et al. performed liquefaction test on five model components, including xylan (hemicellulose), crystalline cellulose, alkaline lignin, soya protein and soybean oil at 290 °C, and found the trend of biocrude yield was highest to lowest as: lipids, protein, cellulose, hemicellulose, and lignin [9].
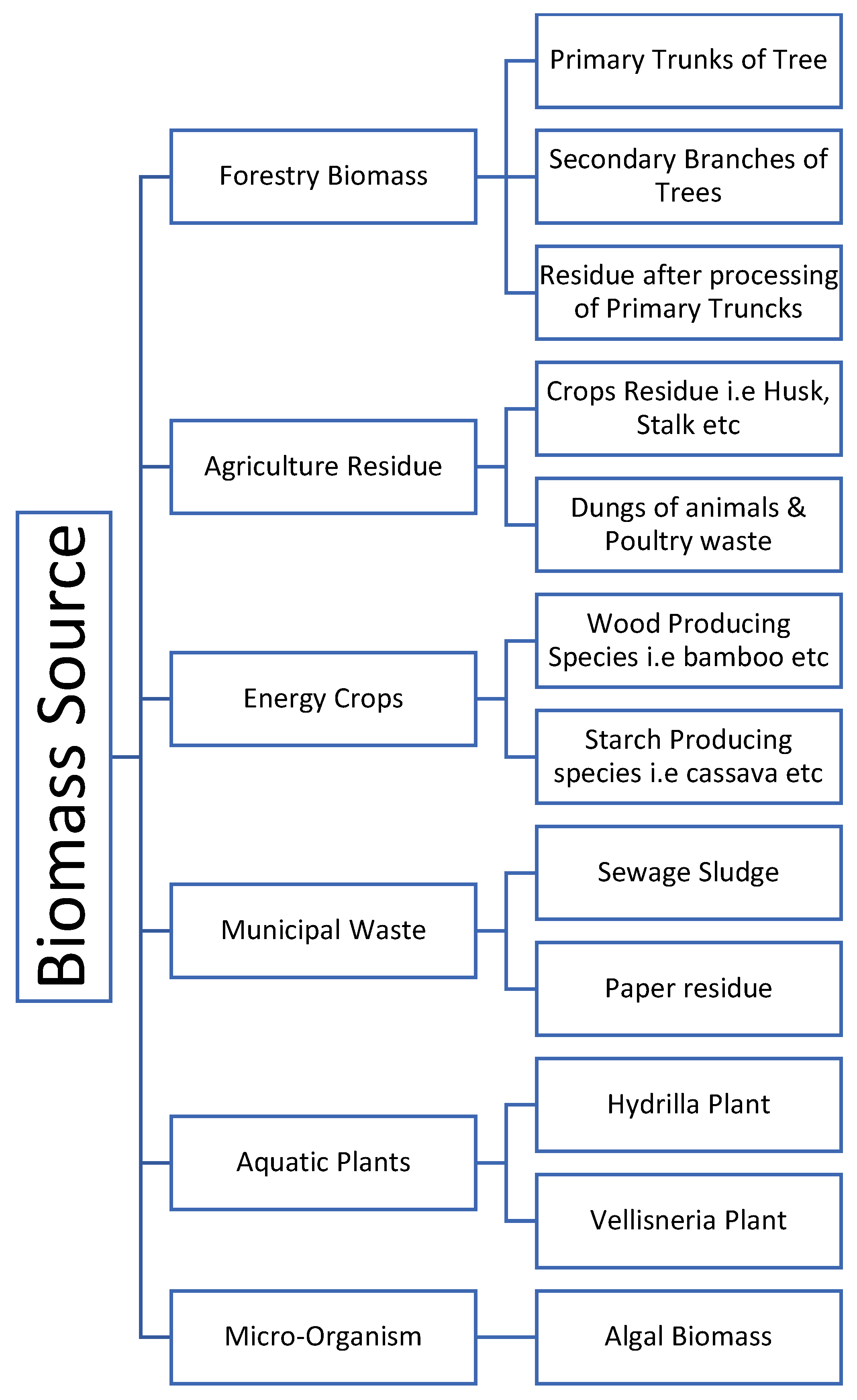
Figure 1. Different biomass for HTL.
2. Process Mode
Batch-type HTL reactors are most common in the literature because of their simple working mechanism [10]. In a batch-type reactor mixture of water, biomass, and catalyst (if needed) are loaded into an autoclave, where it is heated at a certain temperature and selected reaction time; then it is cooled down, and products are collected and analyzed. Any type of material can be screened in an autoclave at different process parameters and operating conditions [11][12]. Conversely, there are some disadvantages of using a batch reactor; for instance, Thermal transience: A batch-type reactor system has to go from ambient conditions to the desired temperature and pressure; hence, process conditions are not constant. This transience makes it difficult to separate the effects of temperature and time. Difficulty in decoupling temperature and pressure: In batch-type experiments, the pressure is dependent on the mixing of reactants; hence, the experimental conditions are often those as of the saturation conditions of the water. The Pre-pressurizing the system with an inert gas can partially overcome this problem [13]. In a continuous system, pressure and temperature can be controlled in a completely independent fashion. Different contact pattern: In a batch-type reactor, the feedstocks are mixed through by an impeller or shaking the reactor [14]. Whereas in a continuous reactor, it is mixed through a continuous stirred flow reactor (CSTR), new feedstocks are continuously supplied, and products are removed. Significant distance toward actual industrial implementation: The industrial application of a batch-type reactor is very limited due to its low production capacity of the products and high energy consumption.
Therefore, it is evident that batch processing alone is not able to provide results that can be directly utilized for the industrial development of the process. Moreover, testing in continuous devices allows for experiencing some technical issues and facts that are typical of continuous processing. One of these is, for example, high-pressure pumping, etc. There are several different studies in the literature that reveal that continuous HTL reactors are available in different sizes, from very small laboratory-scale plants to very large demonstration industrial-scale plants. For instance, the group of Elliot and coworkers at the Pacific Northwest National Laboratories (PNNL) built a continuous batch-type reactor to process different types of biomass with a special focus on the processing of algae macro, microalgae, and residues from agroindustry such as grape pomace. The process diagram is shown in Figure 2. The working mechanism of this plant is described as the slurry is placed in the two pressurized feed tanks by use of a syringe pump (a modified Isco 500D dual system), where the mixture is compressed at a pressure of 200 bar and preheated at 130 °C in a horizontal biocrude jacketed preheater then it is transported to a CSTR reactor, where the mixture reaches the reaction temperature. During this process, the volume of this reactor is altered from 1 L to 400 mL, and plug-flow reactor (PFR) is placed after that to increase the residence time. According to the authors, this alternative approach was adopted to minimize the plugging issues experienced with the PFR reactor, especially when operating with lignocellulosic feedstocks. This setup has also been used for many studies with some adaptations such as different types of algae such as Nannochloropsis sp. [15], macroalga Saccharina spp. [16], and Chlorella, high lipid content [17] and agricultural waste [18], such as grape pomace [19], and wastewater solids [20].
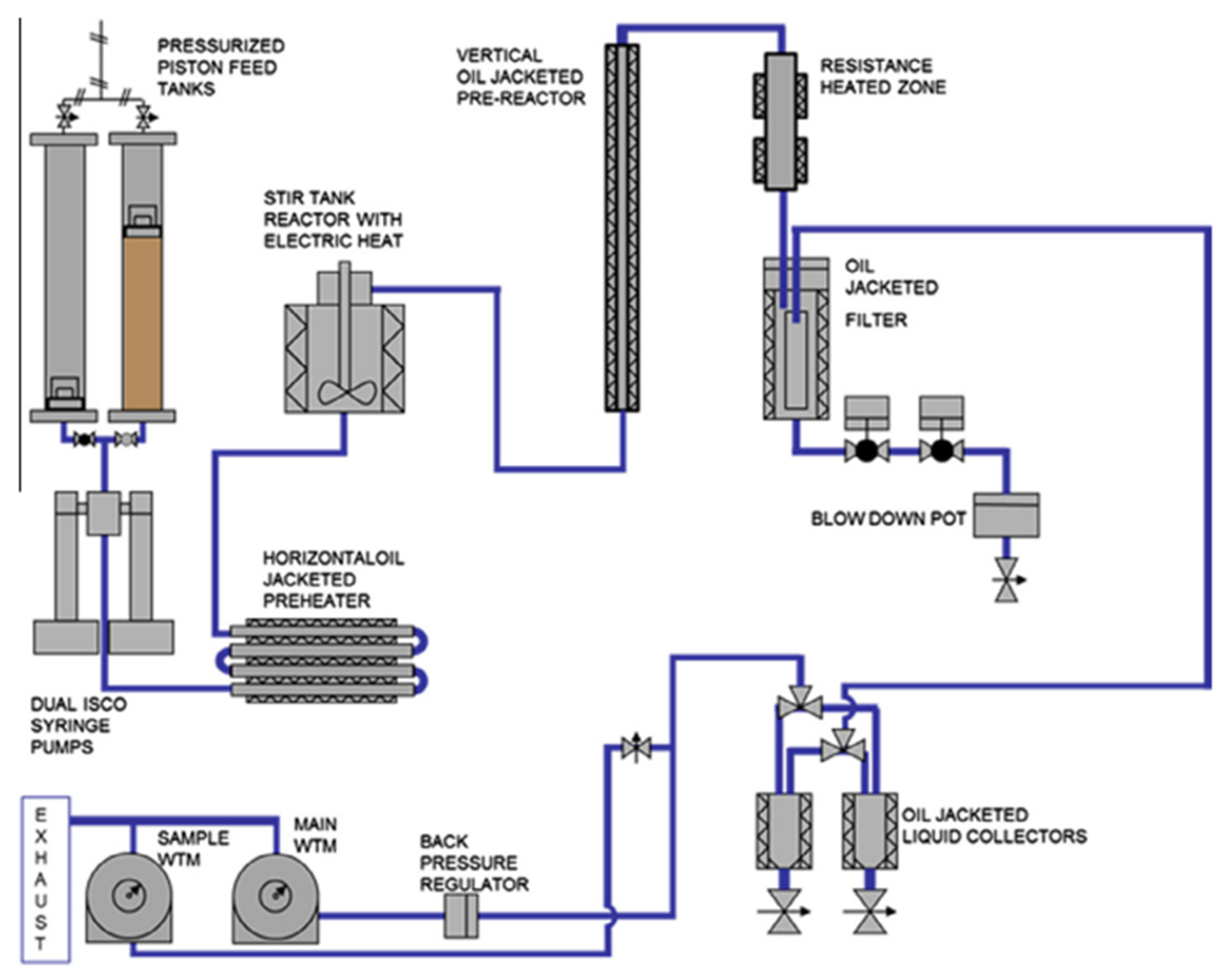
Figure 2. Process scheme of the plant in Pacific Northwest National Laboratories, adopted from [17].
At the University of Sydney, Australia [20], algae, in particular, Chlorella, Spirulina, and Oedogonium, were experimented in a laboratory-based pilot scale plant with a range of 350 °C and 250 bar. All the experiments were conducted at subcritical conditions, with low values of dry matter content (mostly 1–5%, with a few attempts at 10%) and residence times of 3–5 min. The results revealed the maximum biocrude yield of 42% with 10% of dry matter content at a given temperature. At the University of Illinois at Urbana Champaign, USA, Ocfemia et al. [21] established and tested a pilot plant the reactor configuration is CSTR with a residence time of 60 min for the liquefaction of swine manure [21][22]. At Iowa State University, USA, by Suesse et al. [23], the temperature and pressure range of (450 °C and 690 bar) was designed by Supercritical Fluid Technologies Inc. (Newark, Delaware), where different waste streams, specially Rhizopus oligosporus at 270 bar was processed and produced biocrude yields up to 48–61% with an oxygen content of 12–16%. It was also noted that switching from subcritical to supercritical conditions did not lead to considerable change in the yields, whereas the quality of different products reported that similar characteristics of microalgae biocrude were found at 300 °C.
The Kraft lignin (by-product of the pulping industry) has also been used by Chalmers University of Technology (Gothenburg, Sweden) with 0.5 L fixed bed reactor, manufactured at Inconel and packed with pellets of zirconia (ZrO2) [24][25]. This experimental setup consists of a mixture composed by deionized water, lignin, K2CO3 and phenol (which was adopted as a char suppressing agent). The yields of biocrude ranging from 58% to 74%, although the produced biocrude presents a relatively high oxygen content (15–21%) compared to the feed (26%) [24][26].
Leng et al. stated that PFR often achieves better conversion yields than CSTR in a reactor of the same volume. When compared to plug-flow systems, CSTR systems have the inherent disadvantage of mixing mode, which means that some of the feedstock will always be subjected to unfavorable reaction time, some undercooked and some overcooked [27]. This disadvantage exists even though CSTR systems may be easier to operate and maintain, such as cleaning. It is challenging to compare batch and continuous HTL systems because the batch products are frequently equilibrium constrained. Although the yields and heating values of the biocrude produced from swine manure and algae are comparable in continuous modes, the ideal reaction conditions for these two feedstocks in a continuous HTL reactor are very different [28].
It is commonly accepted that for algal biomass, harsher reaction conditions can result in larger yields, lower oxygen content, and higher nitrogen content in the HTL biocrude. Additionally, algal biocrude yields from extremely short residence times in continuous reactors (such as 1–5 min) might be comparable to those from longer residence times in batch modes (such as 60–120 min). Recent batch and continuous HTL investigations also imply that the synthesis of biocrude is favored by faster heating rates and shorter residence times [29].
Through a continuous HTL, this characteristic would also help to increase the energy efficiency and technological viability of biomass-to-fuel systems. Compared to algal feedstocks, continuous HTL of swine dung necessitates a longer retention period. Ocfemia et al. [21][22] performed a 40–80 min continuous HTL of swine dung with a CSTR at 285–325 °C. The best biocrude yield was obtained between 285 and 305 °C for a retention time of 60 min, which is quite close to the results reported in a batch reactor.
According to this continuous HTL investigation, increasing the reaction temperature from 305 °C to 325 °C lowered the biocrude production by 22–25 wt.% at the same residence time, whereas increasing the residence time from 60 to 80 min only increased the biocrude output by 1–2 wt.% at 285–325 °C. The literature states that swine manure has a protein content of 25 wt.% and a carbohydrate content of 35 wt.%, whereas the algal feedstocks used in these continuous HTL studies have a much higher protein content (for example, 60–68 wt.% in Chlorella and Spirulina). Additionally, whereas the continuous HTL of swine manure has been studied in a CSTR, the continuous HTL of algal biomass has been studied in a PFR or in conjunction with a CSTR and PFR [30].
Some continuous bench-scale units are also available. The bench-scale plants are mostly utilized at universities and research institutions for fundamental research or as first experimental devices to obtain data in view of a future scale-up. They are normally of reduced sizes, with reactor volumes often no larger than a few hundred milliliters. One of the first documented studies was carried out at the Karlsruhe Institute of Technology, Germany. Here, a small continuous device was built for the HTL of baker’s yeast and other residual biomass [31][32][33].
A continuous bench-scale unit (CBS1) was established at Aalborg University (Denmark) in collaboration with the Steeper Energy ApS Company. The features of the CBS1 plant are a 10 L tubular reactor designed to operate at supercritical conditions, and an important aspect of this plant is to use of water as recirculating solvent [34][35]. This water phase recirculation enhances the biocrude yield as well as its hydrogen–carbon ratio [13]. In this same study, the author investigated the utilization of glycerol as a co-solvent to reduce the formation of char and to process high organic content.
A continuous HTL pilot plant at Aarhus University (Denmark) has built and commissioned with capacity of around 1 L/min. This HTL plant contains a high-pressure pump to drive the biomass slurry up to 220 bar with maximum temperature of 350 °C, it has also the hydraulic oscillator to increase turbulence in reactor system by achieving better mixing, uniform residence time and to enhance better heat transfer [36]. For better understanding about the all the processes concerning with continuous HTL, the Castello et al. presented a detailed review covering all possible technical and financial aspects in view of existing and future scope of continuous HTL [37].
3. Process Conditions
Process parameters for HTL include reaction temperature, reaction time (also known as retention time), pressure, feedstock/water ratio (solid content), and catalyst applications. The distribution of HTL product yields generated from algal biomass is shown in Figure 3, which summarizes the influence of key process factors (reaction temperature, reaction duration, and total solid content of feedstocks) on that distribution. The most important element influencing HTL product yields and the quality of biocrude produced from feedstocks such as animal dung, algae, and food processing waste has been identified as reaction temperature. Reaction temperatures between 250 °C and 375 °C were typically used to generate biocrude from biomass-containing proteins [38]. The species of the feedstock has a significant impact on the reaction temperature. Microalgae (Chlorella) were converted into biocrude by Yu et al. and Gai et al. without the use of a catalyst, and they proposed that 280–300 °C is the ideal reaction temperature to maximize the yield, heating value, and energy recovery of the biocrude. Using HTL, Brown et al. and Valdez et al. transformed Nannochloropsis into biocrude and concluded that the optimal reaction temperature for achieving the highest biocrude output is between 300 °C and 350 °C. The ideal reaction temperatures vary greatly depending on heating rates, mixing versus nonmixing, and reactor systems (such as batch versus continuous). Therefore, it is crucial to clarify the reaction mechanisms of HTL in terms of the makeup of the feedstock, the reaction temperature, the duration of the reaction, and the catalyst. According to numerous research, more gas products are produced when the HTL reaction temperature is higher than 320 °C. Li et al. discovered that raising the HTL reaction temperature from 320 °C to 380 °C significantly increased the gas product yields. The initial stage’s controlling processes for feedstock are hydrolysis and depolymerization, followed by repolymerization between 220 °C and 375 °C and gasification above 375 °C. More solid residues and char would form as the reaction temperature rose over the reaction regime of repolymerization [39].
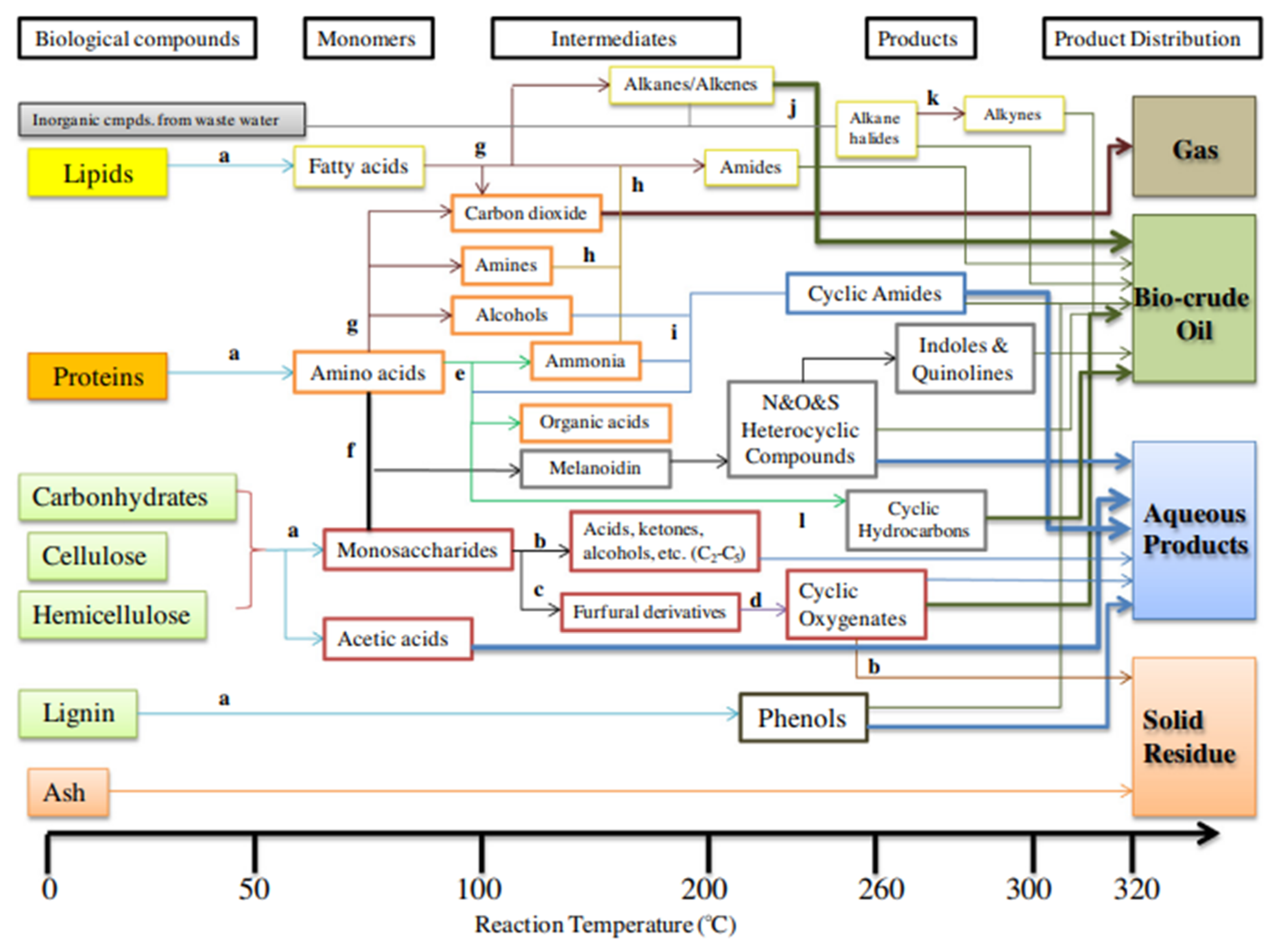
Figure 3. Potential reaction pathways for HTL of AW: (a) hydrolysis; (b) decomposition; (c) dehydration; (d) polymerization; (e) deamination; (f) Maillard reaction; (g) decarboxylation; (h) Aminolysis; (i) cyclization; (j) halogenations; (k) dehydrohalogenation; (l) condensation + pyrolysis. Adopted from [40].
It takes at least 15 min for the reaction for protein-containing biomass, such as swine dung, to produce biocrude compounds resembling asphalt. Algal biomass typically requires 10 min to produce self-separated biocrude products. The reaction kinetics of the HTL process are significantly influenced by reaction time. A maximum biocrude yield can only be achieved with a sufficient reaction period; however, an excessive reaction time will result in the formation of charcoal or gaseous products, lowering the biocrude yield.
During the HTL, additional processes, including condensation and repolymerization, could impact the quality of the biocrude. The effectiveness of the HTL reaction and the conversion of the biocrude could both be considerably impacted by heating rates. Faeth et al. conducted a fast HTL conversion of Nannochloropsis (heating rates as high as 230 °C/min). They reported that an optimal biocrude yield (66 wt.% based on dry ash-free biomass) can be obtained in less than one minute. Biocrude has a comparable carbon content and HHV to those converted from conventional HTL. By using a continuous HTL reactor at 250–350 °C, Jazrawi et al. achieved the greatest algal biocrude output (41.7 wt.%) in 3–5 min. In many HTL tests, the reactor head space was pumped with gas before heating up to create an initial pressure. Applying initial pressure primarily serves to keep water in the liquid phase, lower the enthalpy of water’s phase change, increase the solubility of biomass, and increase energy efficiency. He et al. [41] observed that no biocrude was generated until the initial pressure reached 0.69 MPa when using 0–1.30 MPa of nitrogen gas (N2) as the initial pressure to convert swine dung into biocrude via HTL. However, numerous types of research have shown that further boosting the initial pressure will not increase the output of biocrude. Zhang et al. [42] increased the initial pressure to convert Chlorella into biocrude from 0.69 to 3.45 MPa [43]. It was revealed that beginning pressures above 0.69 MPa had no discernible impact on the yields of HTL products. Yu et al. [44] demonstrated that the HTL product yields converted from Chlorella were not significantly affected by raising the starting pressure from 0 to 0.69 MPa.
On the other hand, Yin et al. transformed cattle dung using an initial pressure of 0–0.69 MPa of CO and N2. When the starting pressure was raised, a declining biocrude output was seen, possibly as a result of the self-condensation reaction that turns the biocrude into solid residues. There are no conclusive findings regarding the impact of the starting pressure on biocrude yields while converting protein-containing biomass via HTL [45].
To stabilize the fragmented products of HTL, reduce free radical condensation, cyclization, and repolymerization, and/or suppress char production, reducing gases have been utilized as the processing gas. The following equations illustrate how H2 can stabilize aromatic radicals (Ar) to produce liquid biocrude products. Using HTL and reducing gases such as CO2 and H2, Yin et al. [46] have turned cow dung into biocrude [47]. They concluded that increasing the biocrude output by 5–15 weight percent can be accomplished by employing CO and H2 as HTL processing gases. He et al. reported similar results when converting swine manure into biocrude by HTL with CO and H2 as processing gases. Despite being expensive and risky alternatives, H2 and CO are excellent at stabilizing fragmented liquefaction products. Synthetic gas (H2/CO), an alternative processing gas, could be another choice to accomplish the same goal [48]. As HTL processing gases, nitrogen, air, and carbon dioxide have all been used. However, other than maintaining reactor headspace pressure and preventing feedstock from gasifying, the function of inert gases in HTL is yet unknown. He et al. showed that utilizing compressed air as the processing gas can achieve a similar biocrude production as those processed with CO2 and N2; however, Yin et al. observed that using air as the HTL processing gas led to a substantially lower biocrude yield than those converted with N2. The relative mass ratio of the processing gas to feedstock was one evident factor in the difference. If the starting gas is too airy, the feedstock can oxidize rather than be transformed into biocrude. One of the crucial factors impacting the output and quality of biocrude is the feedstock’s total solids (TS) level. The types of biomass and the technologies used to gather it have a significant impact on the TS of the feed supply [49]. In contrast, swine dung collected from a flushing system with settling normally has a TS of 5–10 wt.%, and that from a solid floor typically has a TS of 20–30 wt. %. Most research used feedstocks with a TS of 10–30 wt.% to obtain suitable energy and financial returns. An excessively high TS could result in issues such as inadequate heat transmission and material management, such as pumps, while more water would result in higher costs for things such as heating and wastewater treatment. According to Jena et al. [50], the HTL of Spirulina is best achieved with 20 wt.% of TS, and higher TS levels have no discernible impact on product yields. When employing Chlorella as an HTL feedstock, Zhang et al. and Gai et al. reported similar findings [51]. Significantly low biocrude yields (10 wt.%) were obtained when TS was lower than 15 wt.%. The authors hypothesized that in order to amass organic clusters that can be transformed into biocrude, a micro-organic phase may be required as the medium.
4. Catalysis
Under HTL procedures, homogeneous and heterogeneous catalysts have been studied. The various catalysts utilized for HTL of biomass-containing proteins are summarized in Table 1. The catalyst used in HTL did not always produce favorable results. Using heterogeneous catalysts (HZSM-5 and Raney Ni), Zhang et al. transformed Chlorella into biocrude under supercritical ethanol (240–300 °C) without increasing the yield of the biocrude. Similar outcomes were obtained using the catalysts Na2CO3, Co/Mo, CoMo/Al2O3, Ni/Al, Ni/SiO2-Al2O3, Pt/Al, Pd/C, Ru/C, and zeolites to convert microalgae and animal dung into biocrude via HTL [52]. The interaction between liquid, solid, gas, and sub- or supercritical phases in HTL of protein-containing biomass and heterogeneous catalysts is very complex, and as a result, catalyst deactivations and severe intraparticle diffusion limitations may also contribute to the ineffectiveness of catalysts. The biocrude yield in the absence of a catalyst was already close to the upper limit of what is achievable given the restriction of the mass balance and the availability of carbon and hydrogen, which is another explanation for the catalysts’ limited impact on the HTL biocrude yields. It was shown that the HTL biocrude recovered >80% of the C and H atoms from the algal feedstocks without the need for catalysts. The catalyst loadings have an impact on the HTL process. Anastasakis and Ross [53] employed KOH in the range of 5 to 100 weight percent to investigate how catalyst loadings affected the HTL of macroalgae [48]. As catalyst quantities rose from 5 to 100 wt.%, it was noted that the HTL biocrude yields declined by about 10 wt.% while the heating value of biocrude increased by 1.3 MJ/kg [54]. According to Theegala et al., [55] increasing the catalysts’ (Na2CO3) loading from 5 to 20 wt.% did not increase the output of biocrude produced by HTL’s conversion of animal manure. Similar outcomes were also shown when utilizing CaO to convert olive seeds, where the HTL biocrude output declined as the amount of catalysts rose from 5 to 40 wt.%. The HTL biocrude yield appears to benefit from homogeneous catalysts, yet recovering homogeneous catalysts is still difficult. Yu et al. discovered that the algal biocrude output was increased by 5–10 wt.% when they studied the influence of NaOH and Na2CO3 on the HTL of microalgae at 280 °C. Using HTL, Jena et al. [50] found that adding Na2CO3 can increase the output of biocrude by around 7 weight percent. To increase the technoeconomic viability of catalytic HTL conversion of wet biomass, catalyst regeneration has been proposed. Ong [56] has employed NaOH to regenerate catalysts such as Raney Ni when processing used newspapers hydrothermally. Because catalysts are frequently expensive and their manufacture might adversely influence the environment, effective and sustainable techniques to regenerate catalysts for HTL processes are urgently needed. To have a broader look, Table 1 presents changes by different process parameters, especially temperature and catalyst on biocrude properties.
Table 1. Effect of temperature and catalyst on biocrude yield and HHV.
| Feedstocks | Temp (°C) | Catalyst | Non-Cat-Yield (%) | Cat-Yield (%) | Change in Yield (%), by Value | Change in C (%) by Value |
Change in N (%) by Value |
Change in HHV (MJ/kg) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Lignocellulosic biomass | |||||||||
| Wood (birchwood sawdust) | 300 | K2CO3 | 19.11 | 38 | 19 | −4.00 | −0.03 | −3.00 | [57] |
| Wood (birchwood sawdust) | 300 | KOH | 19.11 | 39 | 20 | −5.00 | 0.00 | −3.00 | [57] |
| Wood (birchwood sawdust) | 300 | FeSO4 | 19.11 | 32 | 13 | −4.00 | 0.03 | 2.00 | [57] |
| Wood (birchwood sawdust) | 300 | MgO | 19.11 | 30 | 11 | −5.00 | 0.03 | −2.00 | [57] |
| Oak Wood | 330 | Nickel Powder | 33.12 | 35 | 2 | −1.00 | 0.00 | 0.31 | [58] |
| Eucalyptus | 350 | K2CO3 | 33.12 | 37 | 4 | 3.53 | −0.09 | 1.54 | [59] |
| Eucalyptus | 400 | K2CO3 | 27 | 29 | 2 | −6.50 | −0.15 | 2.93 | [59] |
| Wheat straw | 350 | K2CO3 | 26 | 32 | 6 | 2.61 | −0.19 | 0.54 | [60] |
| Wheat straw | 400 | K2CO3 | 24 | 23 | −1 | −1.18 | 0.27 | −0.47 | [60] |
| Barley straw | 300 | K2CO3 | 18 | 34 | 16 | 5.26 | 0.06 | 2.42 | [12] |
| Animal Manures | |||||||||
| Cow manure | 350 | K2CO3 | 41 | 35 | −6 | 9.00 | 0.90 | 3.00 | [60] |
| Cow manure | 400 | K2CO3 | 32.37 | 32.29 | −0.01 | 4.41 | 0.00 | 0.00 | [60] |
| Swine manure | 350 | K2CO3 | 41 | 37 | −4 | 5.00 | 0.60 | 3.30 | [60] |
| Swine manure | 400 | K2CO3 | 36.97 | 34.76 | −2 | −0.33 | −0.09 | 3.09 | [60] |
| Protein-containing biomass | |||||||||
| Fish sludge | 350 | K2CO3 | 59 | 51 | −8 | 0.92 | 0.17 | 0.50 | [60] |
| Fish sludge | 400 | K2CO3 | 51.27 | 47.17 | −4 | 0.85 | 0.35 | 0.12 | [60] |
| Sewage sludge | 350 | K2CO3 | 40.65 | 45 | 4.45 | 2.51 | −1.02 | 1.30 | [61] |
| Sewage sludge | 400 | K2CO3 | 40.13 | 43 | 2.87 | 1.15 | −1.43 | 0.26 | [61] |
| Sewage sludge | 300 | NiMo/Al2O3 | 27 | 24 | −3 | 4 | 0.03 | 2.42 | [62] |
| Sewage sludge | 300 | CoMo/Al2O3 | 27 | 21 | −6 | 1 | 2.29 | 0.42 | [62] |
| Sewage sludge | 300 | Activated Carbon | 27 | 23 | −4 | 1 | 1.30 | 3.42 | [62] |
| Biopulp (Food waste) | 350 | K2CO3 | 28.9 | 36.6 | 7.5 | 1.24 | −0.09 | 0.80 | [63] |
| Spent compost mushroom | 400 | K2CO3 | 22.86 | 20.42 | −1.8 | −1.47 | −0.65 | −0.67 | [64] |
| Macroalgae (Ulva prolifera) |
280 | MgO | 17 | 16 | −1 | 13 | −0.4 | −2.80 | [65] |
| Macroalgae (Ulva prolifera) |
280 | Al2O3 | 17 | 26 | 9 | 10 | −0.7 | −3.40 | [65] |
| Macroalgae (Ulva prolifera) |
280 | MgCl2 | 17 | 27 | 10 | 11 | −0.2 | 0.60 | [65] |
| Microalgae (Chlorella vulgaris) | 350 | Na2CO3 | 38 | 23 | −15 | 2.90 | −1.60 | 2.00 | [60] |
| Microalgae (Nannochloropsis) | 350 | Na2CO3 | 37 | 21 | −16 | 1.50 | −0.30 | 1.00 | [60] |
| Microalgae (Porphyridium) | 350 | Na2CO3 | 21 | 21 | 0.00 | −26.70 | −2.20 | −12.90 | [60] |
| Microalgae (Nannochloropsis) | 350 | Pd/C | 35 | 57 | 22 | −2 | −0.38 | 0.00 | [66] |
| Microalgae (Chlorella vulgaris) | 300 | NiMo/ Al2O3 | 32 | 29 | −3 | 10.77 | 3.52 | 4.10 | [62] |
| Microalgae (Chlorella vulgaris) | 300 | CoMo/ Al2O3 | 32 | 35 | 3 | 12.19 | 3.49 | 4.97 | [62] |
| Soyabean oil (Triyglycerides) | 320 | KH2PO4 | 85.9 | 93.5 | 8.85 | 1.40 | 0.22 | −1.00 | [67] |
| Soy protein | 320 | K2HPO4 | 28.85 | 31.25 | 2.95 | 0.54 | 0.39 | −0.14 | [68] |
| Potato starch | 320 | K2HPO4 | 11.07 | 22.1 | 11 | 2.60 | −0.01 | 3.04 | [67] |
| Potato starch | 320 | Na2CO3 | 11.07 | 18.77 | 7.6 | −1.32 | 0.00 | 2.14 | [67] |
| Human feces | 330 | Ni-Tm/TiO2 | 40 | 44 | 4 | −4.8 | 1.18 | −4.7 | [68] |
| Human feces | 330 | Tm/ TiO2 | 40 | 40 | 0.0 | −2.6 | 0.47 | −2.7 | [68] |
| Human feces | 330 | Ni/ TiO2 | 40 | 41 | 1 | −3.8 | 0.64 | −2.7 | [68] |
| Human feces | 330 | TiO2 | 40 | 40 | 0.0 | 0.79 | 0.79 | −0.78 | [68] |
5. HTL Products Separation
After the HTL reaction, the HTL products can be divided into four categories: biocrude, solid residue, aqueous products, and gaseous products. A typical separation process for HTL products depends upon the type of feedstock, process mode, and process conditions. At batch scale, the biocrude fraction from HTL products was extracted using organic solvents such as dichloromethane, acetone, and toluene. The effects of various organic solvents on the yields and quality of HTL products have been researched by Valdez et al. [56]. The biocrude produced by Nannochloropsis was recovered using non-polar solvents (hexadecane, decane, hexane, and cyclohexane) and polar solvents (methoxycyclopentane, chloroform, and dichloromethane). It has been discovered that non-polar solvents, including hexadecane and decane, can produce high gravimetric yields of biocrude (39 wt.%). Still, the recovered biocrude has a lower carbon content than that recovered with polar solvents, such as dichloromethane (69 wt.% for decane) (76 wt.%). The solvent used significantly impacts the amount of free fatty acids recovered from the biocrude, with polar solvents recovering more fatty acids than non-polar solvents.
Water and toluene were used sequentially to extract HTL biocrude from wet biowaste and remove nitrogen-containing chemicals from the HTL biocrude. The nitrogen content of algal biocrude was reduced by 16% when an ultrasonically aided water extraction was carried out, but the carbon and hydrogen contents increased. However, alternative extraction methods were also recommended to be considered to increase the effectiveness of extractive denitrogenation and decrease the amount of biocrude lost into the water extract for HTL biocrude. Although HTL biocrude is often recovered using solvents, certain research, especially those concentrating on developing continuous HTL reactors, separate HTL biocrude by decanting the solid products from aqueous products. For instance, no organic solvent was used during the continuous hydrothermal treatment of microalgae in the Pacific Northwest National Laboratory (PNNL) investigations to recover the algal biocrude. Continuous HTL of swine manure uses a similar separating technique.
This entry is adapted from the peer-reviewed paper 10.3390/catal12121621
References
- Toor, S.S.; Conti, F.; Shah, A.A.; Seehar, T.H.; Rosendahl, L.A. Hydrothermal Liquefaction: A Sustainable Solution to the Sewage Sludge Disposal Problem. In Advances in Waste-to-Energy Technologies; CRC Press: Boca Raton, FL, USA, 2019; pp. 143–163.
- Feng, S.; Yuan, Z.; Leitch, M.; Xu, C.C. Hydrothermal liquefaction of barks into biocrude—Effects of species and ash content/composition. Fuel 2014, 116, 214–220.
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Comparative studies of oil compositions produced from sawdust, rice husk, lignin and cellulose by hydrothermal treatment. Fuel 2005, 84, 875–884.
- Kumar, S.; Gupta, R.B. Biocrude production from switchgrass using subcritical water. Energy Fuels 2009, 23, 5151–5159.
- Cheng, L.; Ye, X.P.; He, R.; Liu, S. Investigation of rapid conversion of switchgrass in subcritical water. Fuel Process. Technol. 2009, 90, 301–311.
- Bhaskar, T.; Sera, A.; Muto, A.; Sakata, Y. Hydrothermal upgrading of wood biomass: Influence of the addition of K2CO3 and cellulose/lignin ratio. Fuel 2008, 87, 2236–2242.
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558.
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefinery 2018, 8, 585–595.
- Yang, J.; He, Q.; Niu, H.; Corscadden, K.; Astatkie, T. Hydrothermal liquefaction of biomass model components for product yield prediction and reaction pathways exploration. Appl. Energy 2018, 228, 1618–1628.
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342.
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude production via supercritical hydrothermal co-liquefaction of spent mushroom compost and aspen wood sawdust. Renew. Energy 2017, 111, 392–398.
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292.
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to biocrude: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192.
- Xu, D.; Savage, P.E. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algal Res. 2014, 6, 1–7.
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454.
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Roesijadi, G.; Zacher, A.H.; Magnuson, J.K. Hydrothermal processing of macroalgal feedstocks in continuous-flow reactors. ACS Sustain. Chem. Eng. 2014, 2, 207–215.
- Albrecht, K.O.; Zhu, Y.; Schmidt, A.J.; Billing, J.M.; Hart, T.R.; Jones, S.B.; Maupin, G.; Hallen, R.; Ahrens, T.; Anderson, D. Impact of heterotrophically stressed algae for biofuel production via hydrothermal liquefaction and catalytic hydrotreating in continuous-flow reactors. Algal Res. 2016, 14, 17–27.
- Elliott, D.C.; Schmidt, A.J.; Hart, T.R.; Billing, J.M. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: Grape pomace. Biomass Convers. Biorefinery 2017, 7, 455–465.
- Marrone, P.A.; Elliott, D.C.; Billing, J.M.; Hallen, R.T.; Hart, T.R.; Kadota, P.; Moeller, J.C.; Randel, M.A.; Schmidt, A.J. Bench-Scale Evaluation of Hydrothermal Processing Technology for Conversion of Wastewater Solids to Fuels. Water Environ. Res. 2018, 90, 329–342.
- Jazrawi, C.; Biller, P.; Ross, A.B.; Montoya, A.; Maschmeyer, T.; Haynes, B.S. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013, 2, 268–277.
- Ocfemia, K.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: Development and testing. Trans. ASABE 2006, 49, 533–541.
- Ocfemia, K.S.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure to oil using a continuous reactor system: Effects of operating parameters on oil yield and quality. Trans. ASABE 2006, 49, 1897–1904.
- Suesse, A.R.; Norton, G.A.; van Leeuwen, J. Pilot-scale continuous-flow hydrothermal liquefaction of filamentous fungi. Energy Fuels 2016, 30, 7379–7386.
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.-E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.-I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75.
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.-E.; Vamling, L.; Olausson, L.; Andersson, S.-I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203.
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of Kraft lignin in subcritical water: Influence of phenol as capping agent. Energy Fuels 2018, 32, 5923–5932.
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in biocrude produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020, 401, 126030.
- Chen, P.H.; Quinn, J.C. Microalgae to biofuels through hydrothermal liquefaction: Open-source techno-economic analysis and life cycle assessment. Appl. Energy 2021, 289, 116613.
- Li, S.; Jiang, Y.; Snowden-Swan, L.J.; Askander, J.A.; Schmidt, A.J.; Billing, J.M. Techno-economic uncertainty analysis of wet waste-to-biocrude via hydrothermal liquefaction. Appl. Energy 2021, 283, 116340.
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.-T.; Si, B.; Kang, X.; Zhang, Y. Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour. Technol. 2019, 284, 139–147.
- Hammerschmidt, A.; Boukis, N.; Hauer, E.; Galla, U.; Dinjus, E.; Hitzmann, B.; Larsen, T.; Nygaard, S.D. Catalytic conversion of waste biomass by hydrothermal treatment. Fuel 2011, 90, 555–562.
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Dinjus, E.; Hitzmann, B. Conversion of yeast by hydrothermal treatment under reducing conditions. Fuel 2011, 90, 3424–3432.
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Zevaco, T.; Dinjus, E.; Hitzmann, B. Influence of the heating rate and the potassium concentration of the feed solution on the hydrothermal liquefaction of used yeast and apple pomace under reducing conditions. Biomass Convers. Biorefinery 2015, 5, 125–139.
- Pedersen, T.H.; Grigoras, I.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.; Madsen, R.; Glasius, M.; Arturi, K.R. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl. Energy 2016, 162, 1034–1041.
- Pedersen, T.H.; Jensen, C.U.; Sandström, L.; Rosendahl, L.A. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude. Appl. Energy 2017, 202, 408–419.
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation. Energies 2018, 11, 2695.
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A. Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass Convers. Biorefinery 2021, 1–13.
- Ni, J.; Qian, L.; Wang, Y.; Zhang, B.; Gu, H.; Hu, Y.; Wang, Q. A review on fast hydrothermal liquefaction of biomass. Fuel 2022, 327, 125135.
- Nallasivam, J.; Prashanth, P.F.; Vinu, R. Hydrothermal liquefaction of biomass for the generation of value-added products. Biomass Biofuels Biochem. 2022, 65–107.
- Chen, W.-T.; Zhang, Y.; Zhang, J.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into biocrude. Bioresour. Technol. 2014, 152, 130–139.
- He, J.; Lu, L.; Zhao, C.; Mei, D.; Lercher, J.A. Mechanisms of catalytic cleavage of benzyl phenyl ether in aqueous and apolar phases. J. Catal. 2014, 311, 41–51.
- Zhang, S.; Zhou, S.; Yang, X.; Xi, W.; Zheng, K.; Chu, C.; Ju, M.; Liu, L. Effect of operating parameters on hydrothermal liquefaction of corn straw and its life cycle assessment. Environ. Sci. Pollut. Res. 2020, 27, 6362–6374.
- Leng, L.; Zhang, W.; Chen, Q.; Zhou, J.; Peng, H.; Zhan, H.; Li, H. Machine learning prediction of nitrogen heterocycles in biocrude produced from hydrothermal liquefaction of biomass. Bioresour. Technol. 2022, 362, 127791.
- Yu, G.; Zhang, Y.; Schideman, L.; Funk, T.; Wang, Z. Hydrothermal liquefaction of low lipid content microalgae into biocrude. Trans. ASABE 2011, 54, 239–246.
- Mishra, R.K.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for biocrude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377.
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to biocrude: Effects of conversion parameters on biocrude yield and characterization of biocrude. Bioresour. Technol. 2010, 101, 3657–3664.
- Sawan, S.P.; Sawan, S.P. Supercritical Fluid Cleaning: Fundamentals, Technology and Applications; Elsevier: Amsterdam, The Netherlands, 1998.
- Zhou, X.; Zhao, J.; Chen, M.; Zhao, G.; Wu, S. Influence of catalyst and solvent on the hydrothermal liquefaction of woody biomass. Bioresour. Technol. 2022, 346, 126354.
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Kandasamy, S.; Subramanian, K.P.R.; Purushothaman, K.; Chandrasekaran, A.L.; Narayanan, M. A review on hydrothermal liquefaction of algal biomass on process parameters, purification and applications. Fuel 2022, 313, 122679.
- Jena, U.; Das, K.C.; Kastner, J.R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl. Energy 2012, 98, 368–375.
- Ratha, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal liquefaction of algal feedstocks: The effect of biomass characteristics and extraction solvents. Renew. Sustain. Energy Rev. 2022, 156, 111973.
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481.
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883.
- Deniz, İ. Hydrothermal Liquefaction of Marine Biomass: An Integrated Process. Acad. Platf. J. Eng. Sci. 2018, 6, 36–39.
- Theegala, C.S.; Midgett, J.S. Hydrothermal liquefaction of separated dairy manure for production of biocrudes with simultaneous waste treatment. Bioresour. Technol. 2012, 107, 456–463.
- Ong, M. Evaluation of Anaerobic Membrane Bioreactors and Hydrothermal Catalytic Gasification for Enhanced Conversion of Organic Wastes to Renewable Fuels. Master’s Thesis, University of Illinois Urbana-Champaign, Champaign, IL, USA, 2014.
- Nazari, L.; Yuan, Z.; Souzanchi, S.; Ray, M.B.; Xu, C.C. Hydrothermal liquefaction of woody biomass in hot-compressed water: Catalyst screening and comprehensive characterization of biocrudes. Fuel 2015, 162, 74–83.
- de Caprariis, B.; Bracciale, M.P.; Bavasso, I.; Chen, G.; Damizia, M.; Genova, V.; Marra, F.; Paglia, L.; Pulci, G.; Scarsella, M.; et al. Unsupported Ni metal catalyst in hydrothermal liquefaction of oak wood: Effect of catalyst surface modification. Sci. Total Environ. 2020, 709, 136215.
- Seehar, T.H.; Toor, S.S.; Sharma, K.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Influence of process conditions on hydrothermal liquefaction of eucalyptus biomass for biocrude production and investigation of the inorganics distribution. Sustain. Energy Fuels 2021, 5, 1477–1487.
- Seehar, T.H.; Toor, S.S.; Shah, A.A.; Pedersen, T.H.; Rosendahl, L.A. Biocrude Production from Wheat Straw at Sub and Supercritical Hydrothermal Liquefaction. Energies 2020, 13, 3114.
- Shah, A.A.; Toor, S.S.; Conti, F.; Nielsen, A.H.; Rosendahl, L.A. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504.
- Prestigiacomo, C.; Costa, P.; Pinto, F.; Schiavo, B.; Siragusa, A.; Scialdone, O.; Galia, A. Sewage sludge as cheap alternative to microalgae as feedstock of catalytic hydrothermal liquefaction processes. J. Supercrit. Fluids 2019, 143, 251–258.
- Kohansal, K.; Toor, S.; Sharma, K.; Chand, R.; Rosendahl, L.; Pedersen, T.H. Hydrothermal liquefaction of pre-treated municipal solid waste (biopulp) with recirculation of concentrated aqueous phase. Biomass Bioenergy 2021, 148, 106032.
- Toor, S.S.; Jasiunas, L.; Xu, C.; Sintamarean, I.M.; Yu, D.; Nielsen, A.H.; Rosendahl, L.A. Reduction of inorganics from macroalgae Laminaria digitata and spent mushroom compost (SMC) by acid leaching and selective hydrothermal liquefaction. Biomass Convers. Biorefinery 2018, 8, 369–377.
- Xu, J.; Dong, X.; Wang, Y. Hydrothermal liquefaction of macroalgae over various solids, basic or acidic oxides and metal salt catalyst: Products distribution and characterization. Ind. Crops Prod. 2020, 151, 112458.
- Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61.
- Ding, X.; Mahadevan Subramanya, S.; Fang, T.; Guo, Y.; Savage, P.E. Effects of Potassium Phosphates on Hydrothermal Liquefaction of Triglyceride, Protein, and Polysaccharide. Energy Fuels 2020, 34, 15313–15321.
- Wang, W.; Yang, L.; Yin, Z.; Kong, S.; Han, W.; Zhang, J. Catalytic liquefaction of human feces over Ni-Tm/TiO2 catalyst and the influence of operating conditions on products. Energy Convers. Manag. 2018, 157, 239–245.
This entry is offline, you can click here to edit this entry!
