Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Personal care products (PCPs) have surfaced as a novel class of pollutants due to their release into wastewater treatment plants (WWTPs) and receiving environments by sewage effluent and biosolid-augmentation soil, which poses potential risks to non-target organisms. Among PCPs, there are preservatives that are added to cosmetics for protection against microbial spoilage.
- PCPs
- preservatives
- degradation
1. Characteristics of Preservatives in Cosmetics
The presence of water, a large amount of nutrients, and the way the consumer uses cosmetics promote the proliferation of micro-organisms in selected products. Such impurities can pose a threat to the health of the users and also adversely affect the organoleptic properties of the products [23,24]. In order to prevent microbial growth in cosmetics while extending their shelf life and the period of use of the products, most manufacturers use synthetic preservatives. Because of their biological activity, preservatives present a wide spectrum of undesirable effects for consumers, such as toxicity, irritation, or sensitization. Therefore, the safe use of these compounds is always being called into question. Nowadays, more and more producers decide not to use traditional preservatives in view of the negative public opinion about them. Manufactures who use new and little known chemicals claim that their products are “free” from potentially toxic compounds. Literature data of the possible harmful effects of some preservatives have led to increasing various international regulations and some chemicals have been banned in cosmetic products. In the European Union, the European Chemical Agency (ECHA) created a list of compounds for personal care product preservation from microbial spoilage, according to Annex V, Regulation 1223/2009/EC on Cosmetic Products, as amended by Regulation (EU) 2021/1902, 3 November 2021 [25]. In the United States, the Cosmetic Ingredient Review (CIR), led by a panel of medical experts, collaborates with the US Food and Drug Administration (FDA) to provide a review and assessment of the safety of ingredients used in cosmetics. The regulations regard the type and amount of preservatives added to cosmetics; nevertheless, the regulatory issues concerning preservatives in cosmetics are different in other countries [23,26,27]. It is important to point out that the regulatory status of preservatives is very dynamic and varies from region to region and even from country to country [27,28].
The microbial stability of cosmetic preparations without preservatives is very short; therefore, it is impossible to completely exclude them from cosmetic products manufactured on a large scale. There are some characteristics to take into consideration in preservative selection. The agents should have a broad spectrum and be active against all possible bacteria and fungi. When choosing preservatives, their stability is also very important in a wide range of pH values and temperatures, a lack of interaction with other cosmetic ingredients, and resistance to light and oxygen. Compounds serving as preservatives should be colorless, tasteless, and palpable and should not undergo hydrolysis. All of these features mean that these substances can be considered potentially harmful to the environment and to humans. Furthermore, given their widespread use in daily life, treatment of environmental contamination is challenging, as preservative avoidance may be very difficult to achieve.
Nowadays, most ecotoxicity preservatives, based on the acute toxicity tests performed on aquatic organisms, include organochloride compounds, isothiazolinones, and quaternary ammonium compounds (QACs). This section characterizes the most controversial examples of preservatives used in cosmetics (Table 1).
Table 1. Basic information on the target preservatives.
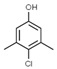
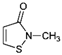
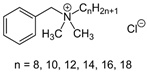
| INCI Name | Triclocarban | Chloroxylenol | Methylisothiazolinone | Benzalkonium Chloride |
|---|---|---|---|---|
| Acronym | TCC | PCMX | MIT | BAC |
| CAS Number | 101-20-2 | 88-04-0/1321-23-9 | 2682-20-4 | 63449-41-2/68391-01-5/68424-85-1/85409-22-9 |
| Formula | C13H9Cl3N2O | C8H9OCl | C4H5NOS | C9H13ClNR (R = C8H17 to C18H37) |
| Molecular weight | 315.58 g mol−1 | 156.61 g mol−1 | 115.1 g mol−1 | - |
| Structure | 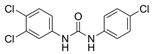 |
 |
 |
 |
1.1. Organochloride Compounds
Organochloride preservatives have rather varying success in the marketplace, ranging from highly controversial and almost banned molecules to those that are very popular and successful. Preservatives from this group include chemicals, such as triclocarban or chloroxylenol. Triclocarban [1-(4-Chlorophenyl)-3-(3,4-dichlorophenyl)urea] (TCC) is a trichlorinated, binuclear phenylurea pesticide that has been globally used as an ingredient in disinfectants, deodorants, soaps, toothpastes, and mouthwashes. Its concentration in products at 0.2% has been approved by the European Union (EU) [29]. TCC has been made and marketed on a massive scale since 1957 and its annual consumption reached 227–454 tons in the USA [30,31,32]. Most commercially obtainable TCC is available in a solid form as a white to off-white crystalline powder with a slight aromatic odor. TCC’s mechanism of action is unknown; however, it is thought to be comparable to that of triclosan (TCS, another antiseptic active component commonly found in PCPs), which has a similar structure [33]. TCS exhibits a biostatic and biocidal efficacy against Gram-positive and Gram-negative bacteria and fungi, as well as against viruses. It permeates the bacterial cell wall and targets multiple cytoplasmic and membrane sites, including RNA synthesis and the production of macromolecules [34]. TCS also blocks the synthesis of fatty acids through the inhibition of enoyl reductase but has no effect on bacterial spores [34,35,36].
Chloroxylenol [4-Chloro-3,5-dimethylphenol] (PCMX) is an antibacterial agent that has been applied to disinfectant products in the United States since the 1950s, such as in liquid soaps, hand washing liquid, solutions used in hospitals to clean surgical instruments, etc. [37]. PCMX is a white to off-white crystalline powder soluble in alcohol, ether, benzene, terpenes, fixed oils, and solutions of alkali hydroxides and it is sparingly soluble in water. Some antibacterial ingredients, such as triclosan in PCPs, have been banned in some countries, leading to an increase in the use of antibacterial alternatives, such as PCMX. The use of PCMX as an antibacterial ingredient in PCPs has been on the rise [38]. PCMX is the active ingredient in Dettol disinfectant solution and has unique in vitro and in vivo antimicrobial activity against Gram-positive and Gram-negative bacteria, fungi, algae, and viruses. The main mechanisms of its action are altering the integrity of membrane proteins, changing the permeability of the cell wall, and disrupting its biological processes. The maximum concentration of PCMX in ready-for-use preparation is 0.5% [39]. The worldwide SARS-CoV-2 pandemic has forced a significant increase in the number of used disinfectants. Increased hygiene standards became applicable not only in hospitals but also in households. Singapore’s National Environment Agency (NEA) prepared a list of active substances that are effective against the virus. One of the active components is chloroxylenol, which has a concentration of 0.12% [40].
1.2. Isothiazolinones
Isothiazolinones are a group of chemicals with antimicrobial effects, which have been used as preservatives in cosmetics, in other consumer products, and in chemical products for occupational use since the 1970s. These compounds are heterocyclic derivatives of 2H-isothiazolin-3-one chemicals containing vicinal sulfur and nitrogen atoms. Isothiazolinones exhibit excellent broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria and fungi at low concentrations and over a wide range of pH values. Due to the sulfur heterocycle, they react with nucleophilic molecules, bind to the thiol groups of proteins and, consequently, inhibit the activity of enzymes that are essential for growth and metabolism, which leads to microbial cell death after a few hours of contact. A number of isothiazolinones exist, which all may be applied to products for occupational use, while only two have been permitted in cosmetic products. These preservatives are often masked under the chemical names of their mixtures: the mixture of methylchloroisothiazolinone with methylisothiazolinone (MCI/MI) in 3:1, also often named by its tradename Kathon™. The cosmetics industry commonly includes Kathon in a wide range of both rinse-off and leave-on formulations, such as shampoos, gels, and hair and skin care products. Methylisothiazolinone (MI, MIT) is one of the most used preservatives in shampoos and one of the most effective. In March 2013, MIT was called the “Allergen of the Year” and its usage has been self-restricted to rinse-off applications. In consumer products other than cosmetics, different isothiazolinones are used, including benzisothiazolinone (BIT) and octylisothiazolinone (OIT) [27,41,42,43,44,45,46].
1.3. Quaternary Ammonium Compounds
Quaternary ammonium compounds (QACs) mainly represent cationic surfactants. In terms of chemical structure, quaternary ammonium compounds belong to ionic compounds that contain four organic groups in the molecule and are associated with nitrogen atoms (including three covalent and one coordination bonds). The antimicrobial activity of QACs depends on the length of the N-alkyl chain, which confers lipophilicity. Benzalkonium chloride (BAC) is one of the most important quaternary ammonium compounds and has been used since 1935 as an antimicrobial additive in various cosmetic preparations at a concentration of 0.1% as well as in pharmaceutical preparations. BAC is a mixture of alkylbenzyl dimethylammonium chlorides with several analogues varying in the length of the aliphatic alkyl chain. In commercial preparations, the aliphatic alkyl chains possess lengths of 12, 14, and 16 carbon atoms. The optimum activity against Gram-positive bacteria and yeast is obtained with chain lengths of 12 to 14 alkyls, while the optimum activity against Gram-negative bacteria is obtained with chain lengths of 14–16 alkyls. Compounds with N-alkyl chain lengths <4 or >18 are virtually inactive. The sensitivity of micro-organisms to the action of QACs also depends on the concentration. At low concentrations (0.5–5 mg L−1), these compounds biostatically act on most bacteria, mycobacteria, spores, fungi, and algae. At medium concentrations (10–50 mg L−1), they show a biocidal effect on bacteria and fungi while, even in very high concentrations, they do not have a biocidal effect on spores, mycobacteria, and prions. Quaternary ammonium salts also act on lipid-enveloped viruses, including HIV (human immunodeficiency virus) and HBV (hepatitis B virus). Thanks to their high antiviral activity, products containing QAC as the active ingredient have been included in the List N: Disinfectants for Use Against SARS-CoV-2, where there are over 500 products meeting the US EPA criteria for the control of SARS-CoV-2 [47,48]. QACs are frequently used at high levels in hair washing and conditioning products because of their anti-static and softening properties. These compounds are widely used not only in cosmetics but also in agriculture (fungicides, pesticides, and insecticides), in health care (medicines), and in industry (anti-corrosive and anti-electrostatic agents) [25,47,49,50,51,52].
2. Microbial Degradation of Preservatives
Human economic activity and progressive urbanization pose a constant threat of environmental contamination with xenobiotics. The presence of cosmetic preservatives in water and soil samples, confirmed by numerous studies, is the cause of many undesirable processes that contribute to the disturbance of the biological balance, as well as the emergence of unfavorable changes at the ecosystem level. Due to the pollution of the natural environment with xenobiotics and their toxic properties, research on their removal has been developing on a large scale and is subject to great interest. However, few literature data that describe the elimination of these preservatives are available. The main way to remove preservatives from the environment is biodegradation carried out by micro-organisms. Biodegradation is a metabolism-dependent process of decomposition of xenobiotics into simpler compounds, taking place with the participation of extracellular and/or intracellular enzymes. This process often transforms the pollutants into simpler compounds that are typically less toxic than the parent compounds. In some cases, biodegradation leads to the mineralization of organic compounds and their degradation into carbon dioxide, water, and/or other inorganic products.
In the scientific literature, few works describe the potential of bacteria and fungi to effectively eliminate triclocarban, chloroxylenol, methylisothiazolinone, and benzalkonium chloride. It should be noted that the process of elimination of xenobiotics, in some cases, is not synonymous with their degradation and detoxification. Table 2 shows the biodegradation of the discussed biocides with the use of micro-organisms.
2.1. Triclocarban
Research studies on the microbial degradation of triclocarban are numerous. The usage of sewage sludge, including of TCC residues in agriculture, poses a serious risk to the environment. The application of micro-organisms over composting biosolids could reduce the environmental risks of the use of sewage sludge as a fertilizer. The biodegradation of TCC via the composting of biosolids under high ventilation resulted in a reduction in xenobiotic concentrations by 83.1% over 16 days [143]. The immobilized microbial cells of Pseudomonas fluorescens (MC46) on biochar could be used for the effective purification of the sewage from TCC. The yield of the process carried out by immobilized P. fluorescens cells was much higher (79.80%) compared to the elimination of TCC by free P. fluorescens cells (42.12%) [144]. Similar results were obtained by Taweetanawanit et al., confirming that TCC was eliminated more efficiently by micro-organisms entrapped in barium alginate [145]. Moreover, researchers observed 3,4-dichloroaniline (34DCA), 4-chloroaniline (4CA), and aniline as by-products emerging via hydrolysis, dehalogenation, hydroxylation, and dechlorination, which were characterized by a lower toxicity than the parent compound. Subsequently, aniline may be transformed through deoxygenation into catechol and it is anticipated that catechol may thereafter undergo ring cleavage [145]. The same bacterial strain was used for the bioaugmentation of TCC-contaminated soil, with an elimination efficiency of 74–76%. P. fluorescens was able to remove TCC as a sole carbon source leading to its detoxification [146]. 34DCA, 4CA, and 4-chlorocatechol were reported to be the major metabolites present as a result of the bacterial degradation of TCC in Sphingomonas sp. YL-JM2C. The formed metabolites and parent compounds were too toxic for the tested strain and inhibited further biodegradation stopping at the level of 35% [147]. Three strains of Ochrobacterium sp. (MC22, TCC-1, and TCC-2) were also capable of triclocarban biotransformation under aerobic and anaerobic conditions [148,149,150,151]. Under aerobic conditions, strain MC22 was able to degrade TCC (initial concentration 9.40 mg L−1) as a sole carbon and energy source with an efficiency of 78% within 6 days and to produce two intermediates 34DCA and 4CA, which were detoxified [148]. Similar metabolites were observed in strains TCC-1 and TCC-2; however, these micro-organisms were characterized by a higher tolerance to upper concentrations of TCC [149,150,151]. The anaerobic degradation of TCC by Ochrobacterium sp. was conducted with acetate as an electron donor. During transformation, 4CA and DCA were formed in all three strains. Only MC22 produced additional aniline [148,149,150]. Moreover, Yun et al. identified a protein accountable for TCC hydrolysis: amidase TccA [149].
2.2. Chloroxylenol
Despite several reports describing the ability of micro-organisms to eliminate chloroxylenol, there is little research devoted to the identification of intermediate products formed during biodegradation and analyzing the mechanisms responsible for the course of these processes. Nowak et al. identified two fungal species capable of degrading chloroxylenol [124]. Cunninghamella elegans IM 1785/21GP and Trametes versicolor IM 373 degraded PCMX with similar efficiencies through different degradation pathways. C. elegans removed 70% of PCMX over 120 h of incubation, at an initial PCMX concentration of 25 mg L−1, via the generation of two metabolites by dehalogenation, aromatic ring hydroxylation, and methyl group oxidation of the parent compound. T. versicolor demonstrated a 79% removal of PCMX over 120 h of incubation via ring opening during hydroxylation, dehalogenation, and oxidation, leading to the formation of three metabolites. The authors suggested that the two different enzyme systems are involved in the initial step of chloroxylenol degradation: cytochrome P450 mono-oxygenases in C. elegans and laccases in T. versicolor. Furthermore, the metabolites generated by tested micro-organisms have a lower toxicity than the parent compound. Among the micro-organisms demonstrating the ability to eliminate PCMX, the microscopic fungus Aspergillus niger was also described in which over 99% of PCMX loss (initial substrate content – 2 mg L−1) was shown after 7 days of incubation [152]. Choi and Oh demonstrated that the removal efficiency of chloroxylenol by activated sludge depended on the initial concentration of xenobiotics [153]. PCMX at a concentration 5 mg L−1 was eliminated over two months with a yield below 50%. In this research, the authors analyzed the impact of PCMX on the bacterial community structure and isolated two bacterial strains probably able to degrade PCMX: Sphingobium and Luteolibacter. Based on the literature data, the authors suggested that the biodegradation of PCMX occurs via dehalogenation and ring hydroxylation.
2.3. Methylisothiazolinone
Little is known about the biodegradation of methylisothiazolinone by micro-organisms. Most often, only the initial stages of the biotransformation of this preservative are known. The few micro-organisms that exhibit the ability to metabolize MIT mainly include various species of filamentous fungi [20,154]. The ability of the ligninolytic fungus Phanerochaete chrysosporium to biodegrade this biocide over a 48 h incubation in liquid culture under aerobic conditions was described. The tested micro-organism was able to completely eliminate MIT at a concentration of 50 µg L−1 and 30 mg L−1 within 12 h [20]. Identified metabolites, formed during the degradation of MIT by P. chrysosporium were mono- and dihydroxylated methylisothiazolinon and N-methylmalonamic acid. The presence of hydroxylated derivatives indicates the involvement of hydroxylating enzymes in the biotransformation process. However, measurements of the activity of laccase, manganese peroxidase, lignin peroxidase, and cytochrome P450 did not confirm the involvement of these enzymes. It is noteworthy that the resulting MIT derivatives are less toxic than the parent compound against D. magna [20]. The process of the biodegradation of MIT by three strains of filamentous fungi, Trichoderma longibrachiatum FB01, Aspergillus niger FB14, and Fusarium solani FB07, occurs differently. Short-chain organic acids, such as tartaric acid, 2-oxobutanoic acid and acetic acid (T. longibrachiatum), malonic acid, 2-oxobutanoic acid, lactic acid, metoxiacetic acid, acetic acid (A. niger) and malonic acid, 2-oxobutanoic acid, propanoic acid, and acetic acid (F. solani) have been identified as metabolites of this preservative. The tested fungi were able to eliminate MIT in 16 h [154]. In both studies, stimulation of the growth of the tested micro-organisms was observed, which probably use this compound as a source of carbon and energy. So far, another pathway for the biodegradation of MIT by the microalgae Scendesmus sp. LX1 has been described. The algae completely removed MIT over 4 days and led to the cleavage of the ring by methylation and carboxylation [155].
2.4. Benzalkonium Chloride
Several reports have already described biologically mediated BAC degradation under laboratory conditions. The first report demonstrated the decomposition of BAC by 20 strains of Burkholderia cepacia bacteria. After an incubation period of 7 days, about 42.6% of BAC was eliminated. Benzyldimethylamine and benzylmethylamine were reported to be the metabolites present at the initial step of BAC degradation as a result of the cleavage of the C–alkyl-N bond. The authors identified two enzymes potentially responsible for C–N bond cleavage: amine oxidase and Rieske-type oxygenase. Moreover, eight catabolic enzymes involved in benzyldimethylamine degradation were identified and the complete degradation of the alkyl group of BAC was noted [156]. The isolation of BAC-degrading micro-organisms from a wide range of ecosystems has been described. Ertekin et al. isolated a strain highly resistant to BAC at a minimal inhibitory concentration of 1024 mg L−1 [157]. The identified Pseudomonas sp. BIOMIG1 was able to eliminate BAC within 3 days by leading to complete mineralization. The evaluation of immobilization as a better method for BAC elimination was described by Bergero et al. [158]. The comparison of BAC biodegradation by planktonic cells of Aeromonas hydrophila MFB03 isolated from industrial WWTPs to its degradation by Ca-alginate-encapsulated cells showed that immobilization increased the efficiency of elimination and, after 48 h, led to the utilization of 90% BAC as a sole carbon and energy source. Due to physical protection, immobilized cells are more resistant to BAC than free cells. Similar results were obtained for Pseudomonas putida ATCC 12633 [159]. Moreover, the use of a microbial consortium formed by these two strains and encapsulated in Ca-alginate is the most efficient method for BAC removal [158]. N,N-dimethylbenzylamine was observed as the result of the C–alkyl-N bond cleavage of BAC-C16 by two isolates from marine sediments, Bacillus niabensis and Thalassospira sp. These bacteria were able to degrade up to 90% BAC over 7 days [160]. Oh et al. studied the biodegradation of BAC as a sole carbon and energy substrate using a microbial community stemming from estuarine sediment and a member of the genus Pseudomonas [161]. Within 12 h, 80% of BAC degradation was observed in a bioreactor inoculated with mixed cultures without the detection of biotransformation products. In order to obtain energy, P. nitroreducens, with the use of amine oxidases, causes the dealkylation of BAC and the formation of two aldehyde products: dodecanal and tetradecanal aldehydes. The obtained metabolites are characterized by a lower toxicity than the parent compound. The aerobic hydroxylation of BAC catalyzed by mono-oxygenase possibly occurs in an enriched community of Pseudomonas spp. The cleavage of the C–alkyl-N bond leads to the formation of benzyldimethylamine. The authors suggested that benzyldimethylamine could be biotransformed by debenzylation to benzoic acid and dimethylamine. These transformations lead to a reduction in acute toxicity (Microtox) [162]. The algal degradation of BAC via pure cultures has been explored in seawater microalgae, Tetrasemis suecica. The tested organisms were able to successfully eliminate BAC-C12 and BAC-C14 from seawater and produced water, with rates of about 100% and 54% within 14 days of incubation, respectively. Furthermore, twelve isomeric intermediates, which are characterized by a lower tendency to be adsorbed into sediments than the parent compounds, were found. The authors suggested that the chemical reactions involved in the biodegradation pathways were multiple hydroxylations followed by dehydration. Hydroxylated BAC-C12 and dihydroxylated BAC-C14 were the most intense by-products formed during BAC-C12 and BAC-C14 transformation, respectively [163].
Table 2. Microbial degradation of cosmetic preservatives.
| Compound | Micro-organism Used | Initial Concentration of Preservative | Removal [%] | Time Taken | Metabolites | References |
|---|---|---|---|---|---|---|
| TCC | Microbial consortium | 975.4 µg kg−1 | 83.1 | 16 d | not analyzed | [143] |
| Pseudomonas fluorescens MC46 (immobilized cells) | 10 mg L−1 | 70.14–79.18 | 24 h | 3,4-dichloroaniline; 4-chloroaniline; aniline; catechol |
[144] | |
| Pseudomonas fluorescens MC46 (free cells) | 10 mg L−1 | 42.12 | 24 h | 3,4-dichloroaniline; 4-chloroaniline; aniline; catechol |
[144] | |
| Pseudomonas fluorescens MC46 (immobilized cells) | 5 mg L−1 10 mg L−1 20 mg L−1 30 mg L−1 40 mg L−1 50 mg L−1 |
73.97 ± 0.03 78.26 ± 0.14 50.98 ± 0.27 27.05 ± 0.71 10.54 ± 0.10 7.88 ± 0.66 |
8 h 8 h 8 h 8 h 8 h 8 h |
Not analyzed 3,4-dichloroaniline; 4-chloroaniline; aniline; Not analyzed Not analyzed Not analyzed Not analyzed |
[145] | |
| Pseudomonas fluorescens MC46 (free cells) | 5 mg L−1 10 mg L−1 20 mg L−1 30 mg L−1 40 mg L−1 50 mg L−1 |
54.52 ± 0.06 44.73 ± 0.20 22.45 ± 0.27 16.98 ± 0.13 6.74 ± 0.01 4.30 ± 0.02 |
8 h 8 h 8 h 8 h 8 h 8 h |
Not analyzed 3,4-dichloroaniline; 4-chloroaniline; aniline; Not analyzed Not analyzed Not analyzed Not analyzed |
[145] | |
| Pseudomonas fluorescens MC46 | 9. 5 mg L−1 | 67 ± 2 | 6 h | Not analyzed | [146] | |
| Sphingomonas sp. YL-JM2C | 4 mg L−1 | 35 | 5 d | 3,4-dichloroaniline; 4-chloroaniline; 4-chlorocatechol; |
[147] | |
| Ochrobactrum sp. TCC-2 | 5 mg L−1 | 56.70 ± 1.50 | 48 h | Not analyzed | [151] | |
| Ochrobactrum sp. MC22 (aerobic conditions) | 9.40 mg L−1 | 78 ± 4.9 | 6 d | 3,4-dichloroaniline; 4-chloroaniline; |
[148] | |
| Ochrobactrum sp. MC22 (anaerobic conditions) | 9.40 mg L−1 | 50% | 14 d | 3,4-dichloroaniline; 4-chloroaniline; aniline; |
[148] | |
| Ochrobactrum sp. TCC-2 (aerobic conditions) | 31.7 μM | 96.88 ± 0.05 | 24 h | 4-chloroaniline; 3,4-Dichloroaniline; |
[149] | |
| Ochrobactrum sp. TCC-2 (anaerobic conditions) | 31.7 μM | 72.70 ± 2.90 | 24 h | 4-chloroaniline; 3,4-Dichloroaniline; |
[149] | |
| PCMX | Cunninghamella elegans IM 1785/21GP | 25 mg L−1 | 70 | 120 h | 2,6-dimethylbenzene-1,4-diol, di-TMS; 2,5-dihydroxy-3-methylbenzaldehyde, di-TMS; |
[124] |
| Trametes versicolor IM 373 | 25 mg L−1 | 79 | 120 h | 4,6-dioxohex-2-enoic acid, TMS; 5-methyl-6-oxohexa-2,4-dienoic acid, TMS; 3-chloro-2,4-dimethylhexa-2,4-dienedioic acid, di-TMS; |
[124] | |
| Aspergillus niger | 2 mg L−1 | 99 | 7 d | Not analyzed | [152] | |
| Klebsiella pneumoniae D2 (free cells) | 8 mg L−1 | 55.7 | 24 h | Not analyzed | [164] | |
| Klebsiella pneumoniae D2 (immobilized cells) | 8 mg L−1 | 88.3 | 24 h | Not analyzed | [164] | |
| Activated sludge | 0.5 mg L−1 | 39.4 ± 17.3 | 72 h | Not analyzed | [153] | |
| Activated sludge | 5 mg L−1 | 49.4 ± 15 | 72 h | Not analyzed | [153] | |
| MIT | Pchanerochaete chrysosporium | 50 µg L−1 and 30 mg L−1 | 100 | 12 h | monohydroxylated MIT; dihydroxylated MIT; N-methylmalonamic acid; |
[20] |
| Trichoderma longibrachiatum FB01 | 10 g L−1 | 100 | 16 h | tartaric acid; 2-oxobutanoic acid; acetic acid; |
[154] | |
| Aspergillus niger FB14 | 10 g L−1 | 100 | 16 h | malonic acid; 2-oxobutanoic acid; lactic acid; metoxiacetic acid; acetic acid; |
[154] | |
| Fusarium solani FB07 | 10 g L−1 | 100 | 16 h | malonic acid; 2-oxobutanoic acid; propanoic acid; acetic acid; |
[154] | |
| BAC | 20 strains of Burkholderia cepacia | 34–64 mg L−1 | 4.7 ± 2.4–42.6 ± 12.3 | 7 d | benzyldimethylamine; benzylmethylamine |
[156] |
| Pseudomonas sp. BIOMIG1 | 200 µM | 62.5 | 3 d | mineralization | [157] | |
| Aeromonas hydrophila MFB03 (immobilized cells) | 25–210 mg L−1 | 90 | 48 h | Not analyzed | [158] | |
| Aeromonas hydrophila MFB03 (free cells) | 50 mg L−1 | 74.2 ± 2.3–80.4 ± 0.6 | 48 h | Not analyzed | [158] | |
| Pseudomonas putida ATCC 12633 (immobilized cells) | 50 mg L−1 | 74 ± 4.70 | 48 h | Not analyzed | [158] | |
| Pseudomonas putida ATCC 12633 (immobilized cells) | 105–315 mg L−1 | 90 | 24 h | Not analyzed | [159] | |
| Bacillus niabensis | 2 mg mL−1 | Up to 90 | 7 d | N,N-dimethylbenzylamine | [160] | |
| Thalassospira sp | 4 mg mL−1 | Up to 90 | 7 d | N,N-dimethylbenzylamine | [160] | |
| Microbial community | 50 mg L−1 | 80 | 12 h | Not detected | [161] | |
| Microbial community | 50 mg L−1 | 100 | 24 h | benzyldimethylamine | [162] | |
| Tetrasemis suecica | 5 mg L−1 | 100 | 3–6 d | OH-BAC-C12; 2OH-BAC-C14 |
[163] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms232214495
This entry is offline, you can click here to edit this entry!
