Microplastics have been documented in nearly every environment on Earth, including deep-sea trenches, freshwater lakes and rivers, groundwater, as well as the atmosphere
inter alia [
1]. The majority of microplastic particles reported in marine, freshwater, and terrestrial biota are microfibers. While there is currently no standard definition for ‘microfiber’, the definition currently proposed by the US National Oceanic and Atmospheric Administration (NOAA) is as follows: microfibers are polymeric fibrous particles (<5 mm) that have been chemically modified and have a length to width aspect ratio of 3:1 [
2]. Microfibers in the environment can be composed of a variety of materials. Synthetic fibers account for nearly 14% of global plastic production [
3], and approximately 60% of textiles are produced using synthetic materials, such as polyester, nylon, polyamide, etc. [
4,
5]. These materials, similar to other microplastics, are derived from fossil fuels and sometimes feedstocks consisting of recycled content.
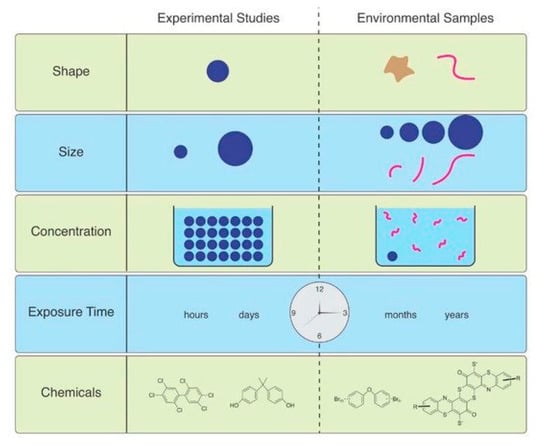
While most microplastics documented in environmental samples are microfibers, the majority of experimental studies related to the effects of microplastics expose organisms to microspheres (or beads), pellets, or fragments, which can be easily purchased at specified sizes and polymer types [
16]. Far fewer studies have used microfibers [
16,
17,
18,
19]. When the effects of microfibers are compared to those of non-fibrous particles (i.e., spheres, fragments, pellets), fibers tend to be more toxic [
16]. Further, most studies on microfibers have focused on the effects of synthetic fibers, whereas the impacts of natural and semi-synthetic fibers are understudied. Yet, when investigated, natural and semi-synthetic fibers have comparable effects to those of their synthetic counterparts [
20,
21]. Furthermore, many experimental studies on fibers use exposure concentrations considerably higher than those found in the environment and tend to expose organisms for short periods of time. Further, given the discrepancy between the conditions used in experimental studies and conditions that occur in the environment, it is difficult to make conclusions about the actual effects of microfibers (
Figure 1) [
22].
Microfibers can vary in the polymeric materials of which they are composed, as well as the suite of chemicals that are intentionally added during production (i.e., chemical additives, dyes, and finishes) and unintentionally accumulated from the environment (i.e., persistent environmental contaminants) [
23,
24,
25,
26]. Many of these chemicals are known to be carcinogenic, mutagenic, and/or endocrine-disrupting compounds (EDCs) and can potentially leach from fibers to the surrounding environment [
12,
27]. Once in the environment, persistent environmental contaminants, such as heavy metals, PCBs, PAHs, can adsorb to fibers, causing “weathered” (or environmentally exposed) fibers to have different associated chemical profiles and therefore different toxicity than “virgin” fibers [
28]. Given their high surface area to volume ratio and demonstrated high sorptive capacity for contaminants [
24], microfibers may be a vector for chemical exposure in biota.
2. Microfibers as Vectors for Chemical Exposure
While known effects of microfibers are described above, these studies do not delineate physical effects of the fibers themselves from potential impacts of associated chemical contaminants [
97]. Microfibers, including semi-synthetic and natural materials, present a complex mix of physical and chemical properties that can influence toxicity. These include the material (i.e., polymeric) composition of the particle, the size, shape, density, and surface properties of the particle, as well as the profile of associated chemical contaminants [
98,
99].
The textile industry is regarded as one of the most chemical-intensive industries on the planet. Thousands of chemicals are registered for use in the production, assembly, storage, and shipping of textile fibers, including dyes and finishing products [
23,
100,
101]. Not only are chemicals intentionally incorporated into textile fibers during production and use, chemical contaminants (e.g., PCBs, PBDEs) may also be unintentionally sorbed from the surrounding environment [
24,
102,
103]. Here, we explored a major outstanding question pertaining to the ecological and human health impacts of microfibers: the capacity of microfibers to act as vectors for toxic chemical exposure.
2.1. Chemical Usage and Accumulation on Textile Fibers
Chemicals are used throughout the textile production process, from the harvesting of raw materials to finishing and storage. The application of synthetic materials used in the production of plastic textile fibers begins with the extraction of fossil fuels and the manufacturing of plastic monomers. Polymer products, used to create synthetic textile fibers, are created from monomers via polymerization reactions. Following their creation, it is possible for unreacted monomers and intentionally added substances (e.g., titanium (III) chloride, antimony), which drive polymerization reactions, to remain on finished polymer products. Additionally, non-intentionally added substances, including reaction byproducts, degradation products, and contamination, may be incorporated into synthetic polymer products. Despite being derived from natural sources, semi-synthetic and natural textile fibers also undergo heavy chemical processing and thus cannot be considered inherently natural or environmentally friendly [
104]. These include substances used in the cultivation of plant and animal fibers (e.g., herbicides, insecticides, rodenticides), as well as the chemical processing used to create regenerated or semi-synthetic fibers [
105].
While many chemicals are used in the cultivation and synthesis of textile fibers themselves, the bulk of chemicals used during production are applied to constructed garments. These include pigments and dyes, wrinkle-resistance finishing, antimicrobial agents, and water and stain repellents [
23,
106]. Many of the finishing products that are applied to textiles are persistent, bioaccumulative, and toxic (PBT) substances and were identified as chemicals of concern due to their potential impacts on human and environmental health [
107]. The largest and most diverse group of chemicals applied to textiles during production are pigments and dyes (i.e., colorants). Human health effects of these chemicals vary and can include allergic reaction, growth and developmental impacts, as well as carcinogenic effects [
108]. Further, through contact with skin, colorants such as azo dyes can produce carcinogenic degradation compounds [
109,
110]. Azo dyes are the most dominant dye class used in textile production, accounting for 60–70% of the global market [
111].
Other known toxicants used in the production of textile fibers include per- and poly-fluorinated alkylated substances (PFOS and PFAS), which are applied to textiles and other consumer products (e.g., food packaging, cookware). Phthalates are another group of chemicals commonly applied to textiles, most often used in polyvinyl chloride (PVC) prints and the coatings of decorative images [
112]. Phthalates are well known endocrine-disrupting compounds (EDCs) due to their harmful impacts on reproductive health [
113]. The relative importance of microfibers as a source and vector of these chemicals in the environment is not yet quantified, but the textile industry is suspected to be a significant source, e.g., via effluent and water discharge [
7,
8].
Finally, there are also chemical contaminants that may unintentionally accumulate on our garments from the surrounding environment [
24,
102,
103]. The sorption and desorption of chemicals is dictated by their physical-chemical properties, as well as the physical-chemical properties of the textiles [
114]. Chemicals, including those not originally intended for use in textiles, such as PBDEs, phthalates, organophosphate esters (OPEs), polycyclic aromatic hydrocarbons (PAHs), and PCBs, have been documented to accumulate on clothing from contact with air, dust, and/or contaminated products. In fact, textiles have a large sorption capacity for semi-volatile organic compounds (SVOCs) [
24,
102,
103]. It is estimated that the amount of clothing worn by an adult (2 m
2) can sequester the equivalent of approximately 100 m
3 of air per day (Saini et al. 2016). Further, microfibers released from textiles have been shown to adsorb chemicals, including PAHs and PCBs [
115,
116,
117].
Although we primarily focused on microfibers derived from textiles here, other sources of microfibers exist, but they are not as well characterized. These include carpeting and personal care products [
12,
13,
14,
15]. Cigarette filters, composed of cellulose acetate, a semi-synthetic fiber [
12,
118], are a major non-textile source of microfibers. It is estimated that the 4.5 trillion cigarette filters littered annually generate approximate 0.3 million tons of microfibers each year [
12,
119]. A suite of toxic chemicals is associated with cigarette filters, including heavy metals, PAHs, etc., and cigarette filter leachates are well known to be toxic [
120,
121,
122]. Further, Belzagui et al. [
12] found that negative effects of cigarette leachate can be exacerbated by the physical effects of the fibers.
Bioplastics such as PLA are offered as a “green” alternative to synthetic and semi-synthetic plastics due to their ability to biodegrade under certain industrial conditions [
123] and constitute another suite of understudied semi-synthetic materials for which little is known about their environmental fate and effects. Though bioplastics have not been heavily utilized in textile production, interest is growing among smaller producers such as Xtep. Studying the effects of bioplastics is important to addressing emerging synthetic alternatives and their environmental effects.
2.2. Microfiber-Mediated Chemical Release and Exposure
While chemicals associated with microfibers can broadly be categorized as those that are (1) intentionally added during production and use and those that (2) unintentionally accumulate from the environment, most studies investigating the chemical sorption-desorption dynamics of synthetic microfibers focused on the latter category of chemicals. These include pharmaceuticals, heavy metals, and organic contaminants (e.g., PAHs, PCBs) [
115,
124,
125,
126]. While most research on the sorption behaviors of environmental contaminants to microplastics has focused on microplastic fragments or spheres [
127,
128,
129], a few studies demonstrated the sorption and release of chemicals from synthetic and natural textile fibers [
28,
116,
117,
130,
131].
Several factors likely influence the sorption and desorption of chemicals to microfibers, including the physical properties (i.e., crystallinity, surface area, surface condition) and chemical properties (i.e., polymer type, surface charge, hydrophobicity) of the fiber, as well as the physical-chemical properties of the chemical and surrounding environmental media [
116,
132]. Additionally, the degree of physical weathering of a fiber in the environment may influence its surface morphology and associated chemical profile [
28]. Microfibers, including natural, semi-synthetic, and synthetic fibers, degrade in the environment via photo-degradation. Sait et al. [
28] demonstrated that the degree of weathering (measured as changes to surface morphology and fragmentation) varied among different types of fiber (i.e., polyester, acrylic, wool). Further, they identified chemical leachates, including monomers, additives, and degradation products, in both pristine and degraded fibers.
2.3. Toxicity of Virgin vs. ‘Weathered’ Particles
To date, empirical evidence demonstrating the potential importance of microfibers as vectors and/or sources of chemicals to biota remains a major understudied question in the microfiber and microplastics research fields (see [
133,
134]). Chemical profiles vary between virgin and ‘weathered’ microplastics, including fibers [
28,
135,
136]. While research on the toxicity of virgin versus ‘weathered’ fibers is limited, this type of exposure study is critical for delineating between the physical effects of the fibers and their associated chemical profile. Nearly all ecotoxicological testing of this type has investigated the toxicity of virgin versus ‘weathered’ non-fibrous particles, such as pellets and spheres. These studies suggest that organisms respond differently when particles are exposed to the environment, such that ‘weathered’ particles sometimes cause greater toxicity compared to virgin particles [
137,
138,
139,
140,
141]. However, sorption-desorption can differ between non-fibrous microplastics and fibers given differences in physio-chemical properties, sorptive capacities, and chemical profiles [
128]. Further, other factors that influence the uptake of chemicals from ingested particles, such as gut residence time, can also vary between fibers and other types of microplastics. Another important consideration for future investigations is the bioavailability of microfiber-sorbed contaminants compared to other exposure pathways [
136,
142,
143]. For example, Beckingham and Ghosh [
142] demonstrated that the PCB uptake from microplastic spheres into benthic worms was much lower than the uptake from surrounding sediments. Further, Thaysen et al. [
143] reported evidence of the bidirectional transfer of PBDEs from ingested microplastics in seabirds, where in some cases highly contaminated tissues may be a source of contaminants to ingested microplastics. In these cases, microplastics were not a significant vector for chemical exposure. Given the diversity of microplastics, microfibers, and their associated chemical contaminant profile, generalization to this entire contaminant class requires further research.