Secretoneurin (SN) is a 33 amino-acid evolutionary conserved neuropeptide from the chromogranin peptide family. SN’s main effects may be cardioprotective and are believed to be mediated through its inhibition of calmodulin-dependent kinase II (CaMKII), which influences intracellular calcium handling. SN inhibition of CaMKII suppresses calcium leakage from the sarcoplasmic reticulum through the ryanodine receptor. This action may reduce the risk of ventricular arrhythmias and calcium-dependent remodelling in heart failure. SN is also involved in reducing the intracellular reactive oxygen species concentration, modulating the immune response, and regulating the cell cycle, including apoptosis. SN can predict mortality in different disease states, beyond the classical risk factors and markers of myocardial injury. Plasma SN levels are elevated soon after an arrhythmogenic episode. In summary, SN is a novel biomarker with potential in cardiovascular medicine, and probably beyond.
- secretoneurin
- ryanodine receptor
- heart failure
- arrhythmogenesis
1. Basic Properties of Secretoneurin
2. Biological Effects of Secretoneurin
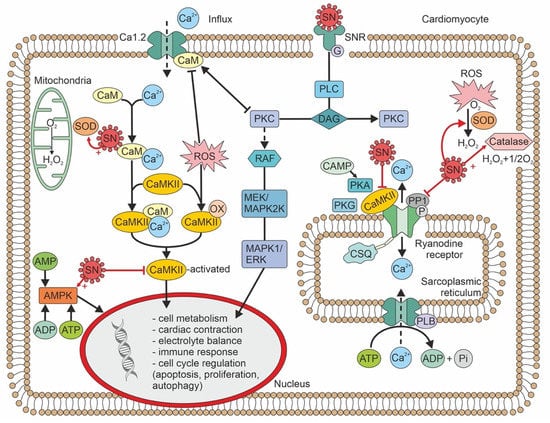
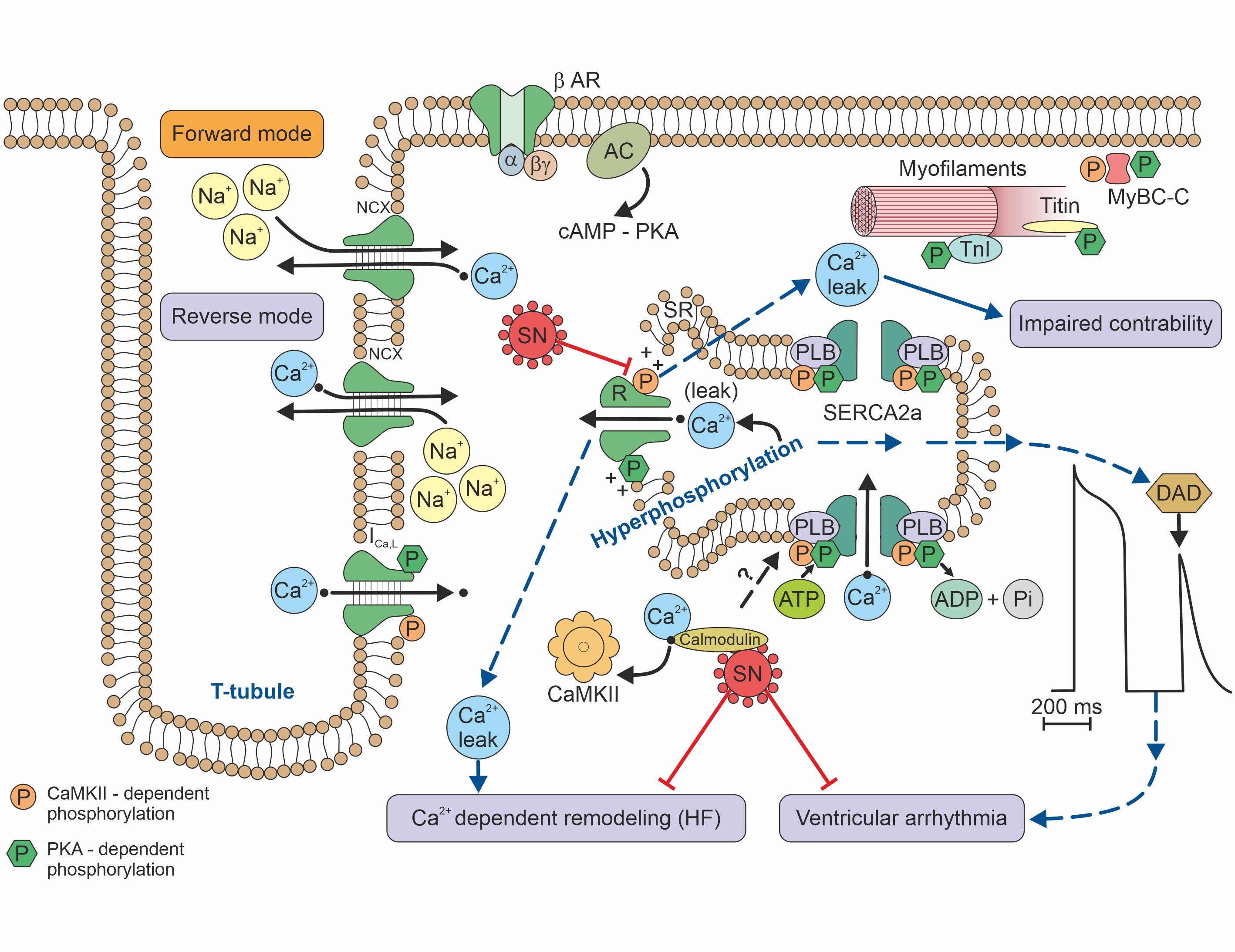
Figure 2. Calcium handling/signalling in a ventricular cardiomyocyte illustrates the potential involvement of SN in arrhythmogenic risk, heart failure, and calcium-dependent remodelling
3. Cellular Pathophysiology of Secretoneurin
This entry is adapted from the peer-reviewed paper 10.3390/jcm11237191
References
- Trudeau, V.L.; Martyniuk, C.J.; Zhao, E.; Hu, H.; Volkoff, H.; Decatur, W.A.; Basak, A. Is secretoneurin a new hormone? Gen. Comp. Endocrinol. 2012, 175, 10–18.
- Zhao, E.; Hu, H.; Trudeau, L.V. Secretoneurin as a hormone regulator in the pituitary. Regul. Pept. 2010, 165, 117–122.
- Kirchmair, R.; Hogue-Angeletti, R.; Gutierrez, J.; Fischer-Colbrie, R.; Winkler, H. Secretineurin: A neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 1993, 53, 359–365.
- Zhao, E.; Basak, A.; Crump, K.; Trudeau, V.L. Proteolytic processing and differential distribution of secretogranin-II in goldfish. Gen. Comp. Endocrinol. 2006, 146, 100–107.
- Morvan, J. Tooze SA. Discovery and progress in our understanding of the regulated secretory pathway in neuronedocrine cells. Histochem. Cell Biol. 2008, 129, 243–252.
- Pertl, C.; Kaufmann, W.; Amann, R.; Heinemann, A.; Ebeleseder, K.; Polansky, R.; Kim, S. Secretoneurin, a novel neuropeptide, in the human dental pulp. Arch. Oral Biol. 1998, 43, 361–365.
- Jansson, A.M.; Røsjø, H.; Omland, T.; Karlsson, T.; Hartford, M.; Flyvbjerg, A.; Caidahl, K. Prognostic value of circulation chromogranin A levels in acute coronary syndromes. Eur. Heart J. 2009, 30, 25–32.
- Røsjø, H.; Masson, S.; Latini, R.; Flyvbjerg, A.; Milani, V.; La Rovere, M.T.; Revera, M.; Mezzani, A.; Tognoni, G.; Tavazzi, L.; et al. Prognostic value of chromogranin A in chornic heart failure: Data from the GISSI-Heart failure trial. Eur. Heart J. Fail. 2010, 12, 549–556.
- Røsjø, H.; Husberg, C.; Dahl, M.B.; Stridsberg, M.; Sjaastad, I.; Finsen, A.V.; Carlson, C.R.; Øie, E.; Omland, T.; Christensen, G. Chromogranin B in heart failure: A putative cardiac biomarker expressed in the failing myocardium. Circ. Heart Fail. 2010, 3, 503–511.
- Fischer-Colbrie, R.; Kirchmair, R.; Kahler, C.M.; Wiedermann, C.J.; Saria, A. Secretoneurin: A new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr. Pept. Sci. 2005, 6, 373–385.
- Steiner, R.; Colbrie-Fischer, R.; Bletsa, A.; Laimer, J.; Troger, J. Secretoneurin and PE-11 immunoreactivity in the human dental pulp. Arch. Oral Biol. 2018, 86, 13–17.
- Mitchell, K.; Mikwar, M.; Da Fonte, D.; Lu, C.; Tao, B.; Peng, D.; Erandani, W.U.; Hu, W.; Trudeau, V.L. Secretoneurin is a secretogranin-2 derived hormonal peptide in vertebrate neuroendocrine systems. Gen. Comp. Endocrinol. 2020, 299, 113588.
- Ottesen, A.H.; Louch, W.E.; Carlson, C.R.; Landsverk, O.J.; Kurola, J.; Johansen, R.F.; Moe, M.K.; Aronsen, J.M.; Høiseth, A.D.; Jarstadmarken, H.; et al. Secretoneurin is a novel prognostic cardiovascular biomarker associated with cardiomyocyte calcium handling. J. Am. Coll. Cardiol. 2015, 65, 339–351.
- Ottesen, A.H.; Carlson, C.R.; Eken, O.S.; Sadredini, M.; Myhre, P.L.; Shen, X.; Dalhus, B.; Laver, D.R.; Lunde, P.K.; Kurola, J.; et al. Secretoneurin is an Endogenous CaMKII Inhibitor that Attenuates Ca2+-Dependent Arrhythmia. Circ. Arrhythm. Electrophysiol. 2019, 12, 007045.
- Kirchmair, R.; Gander, R.; Egger, M.; Hanley, A.; Silver, M.; Ritsch, A.; Murayama, T.; Kaneider, N.; Sturm, W.; Kearny, M.; et al. The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 2004, 109, 777–783.
- Schgoer, W.; Theurl, M.; Albrecht-Schgoer, K.; Jonach, V.; Koller, B.; Lener, D.; Franz, W.M.; Kirchmair, R. Secretoneurin gene therapy improves blood flow in an ischemia model in type 1 diabetic mice by enhancing therapeutic neovascularization. PLoS ONE 2013, 8, e74029.
- Albrecht-Schgoer, K.; Schgoer, W.; Holfeld, J.; Theurl, M.; Wiedemann, D.; Steger, C.; Gupta, R.; Semsroth, S.; Fischer-Colbrie, R.; Beer, A.G.; et al. The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 2012, 126, 2491–2501.
- Assefa, F.; Lim, J.; Kim, J.A.; Ihn, H.J.; Lim, S.; Nam, S.H.; Bae, Y.C.; Park, E.K. Secretoneurin, a neuropeptide, enhances bone regeneration in a mouse calcarial bone defect model. Tissue Eng. Regen. Med. 2021, 18, 315–324.
- Kahler, C.M.; Schratzberger, P.; Wiedermann, C.J. Response of vascular smooth muscle cells to the neuropeptide secretoneurin. A functional role for migration and proliferation in vitro. Arter. Thromb. Vasc. Biol. 1997, 17, 2029–2035.
- Tanaka, K.; Honda, M.; Takabate, T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J. Am. Coll. Cardiol. 2001, 37, 676–685.
- Cingolani, H.E.; Ennis, I.L.; Aiello, E.A.; Pérez, N.G. Role of autocrine/paracrine mechanisms in response to myocardial strain. Pflug. Arch. 2011, 462, 29–38.
- Chen, H.-L.; Liu, Y.; Jiang, W.; Wang, X.-X.; Yuan, G.-L.; Zhao, Y.-L.; Yu, C. Secretoneurin suppresses cardiac hypertrophy through suppression of oxidant stress. Eur. J. Pharmacol. 2018, 822, 13–24.
- Chan, C.K.Y.; Vanhoutte, P.M. Secretoneurin facilitates endothelium-dependent relaxations in porcine coronary arteries. Am. J. Physiol. Circ. Physiol. 2011, 300, H1159–H1165.
- Kähler, C.M.; Schratzberger, P.; Kaufmann, G.; Hochleitner, B.; Bechter, O.; Götsch, C.; Wöll, E.; Marschang, P.; Herold, M.; Wiedermann, C.J. Transendothelial migration of leukocytes and signaling in response to neuropeptide secretoneurin. Regul. Pept. 2002, 105, 35–46.
- Reinisch, N.; Kirchmair, R.; Kähler, C.M.; Hogue-Angeletti, R.; Fischer-Colbrie, R.; Winkler, H.; Wiedermann, C.J. Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett. 1993, 334, 41–44.
- Zhao, E.; Zhang, D.; Basak, A.; Trudeau, V.L. New insights into granin-derived peptides: Evolution and endocrine roles. Gen. Comp. Endocrinol. 2009, 164, 161–174.
- Ischia, R.; Gasser, R.W.; Fischer-Colbrie, R.; Eder, U.; Pagani, A.; Cubeddu, L.X.; Lovisetti-Scamihorn, P.; Finkenstedt, G.; Laslop, A.; Winkler, H. Levels and molecular properties of secretoneurin-immunoreactivity in the serum and urine of control and neuroendocrine tumor patients. J. Clin. Endocrinol. Metab. 2000, 85, 355–360.
- Stridsberg, M.; Eriksson, B.; Janson, E.T. Measurement of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul. Pept. 2008, 148, 95–98.
- Taupenot, L.; Harper, K.L.; O’Connor, D.T. The chromogranin-secretogranin family. N. Engl. J. Med. 2003, 348, 1134–1149.
