Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Tumor organoids are defined as self-organized three-dimensional assemblies of heterogeneous cell types derived from patient samples that mimic the key histopathological, genetic, and phenotypic characteristics of the original tumor. This technology is proposed as an ideal candidate for the evaluation of possible therapies against cancer, presenting advantages over other models which are currently used.
- 3D cell culture
- cancer
- extracellular matrix
- organoids
- tomoroids
1. Organoids and Their Production
Three-dimensional cell culture is mainly represented by spheroids and organoids, the spheroids are simple structures, made up of only one type of cell, while the organoids are three-dimensional structures with complexity and heterogeneous cellular conformation, which mimic the morphology and physiology of their tissue of origin (recapitulation). The above is achieved in conditions that allow the cells that form the organoid to achieve self-renewal and provide mitogenic stimuli (culture medium that provides nutrients, growth factors that allow cell signaling, and extracellular matrix that provides support and adhesion of cells. Organoids can be initiated from embryonic stem cells, somatic adult stem cells, and induced pluripotent stem cells, although they can also originate from primary cultures of cancerous tissue samples [36]. Tumor-like organoids (also called tumoroids) are also defined as self-organized 3D assemblages of neoplastic cells derived from patient-specific tissue samples, which mimic the key histopathological, genetic, and phenotypic characteristics of the original tumor [14].
Different types of organoids have been generated from immortalized cell lines; for example, pancreatic cancer cell lines expressing the membrane marker (FC1245) [12] have been used individually or in co-culture with another line of pancreatic cancer, which express the GFP (green fluorescent protein) marker seeded in Matrigel to generate individual organoids [37]. Organoids have also been established from different types of breast cancer cell lines or primary cultures in Matrigel [38]. The above systems, with the appropriate growth factors, grow and differentiates to simulate the structure of a human tumor. In clinical research, the use of patient-derived organoids has great relevance for personalized medicine [39,40,41] For example, cells can be obtained from solid or liquid biopsies and used to generate a primary culture in a 3D matrix [14,42]. The solid biopsy has shown greater success in obtaining organoids. The tissue is digested enzymatically and/or mechanically, and is seeded on the matrix that will support the organoid. In this tissue, several types of cells that make up the tumor can be obtained, which contributes to preserving its heterogeneity [43].
Conversely, liquid biopsies have the advantage of the presence of circulating tumor cells (CTCs), which have markers and physical elements responsible for tumor spread and metastasis [44]. However, growing cultures derived from CTCs may be slow, given the low concentration of cells present in these fluids. Organoids have been generated from CTCs for pancreatic, breast, gastric, colon, and other cancers [45,46,47,48,49,50]. After the isolation of the solid or liquid biopsies, they are seeded in a biomaterial that allows biomimetics of the extracellular matrix (ECM).
2. Techniques for the Development of Tumor Organoids
The initial conditions of the organoid contribute to its variability, including the initial cell population, its positioning and aggregation [135], its niche or extracellular environment, its physicochemical characteristics, and its culture conditions. At first, organoid cultures may start as spheroids or agglomerated cells. After cell differentiation, promoted by growth factors, they acquire their organoid characteristics [136]. These, once spread, are maintained under controlled CO2 and temperature conditions (5% v/v of CO2 and 37 °C). In systems used for organoids, they are submerged in culture medium and microfluidics, and cultured in an air–liquid interface, or in bioreactors [13].
2.1. Submerged Culture in Scaffolding
The first method consists of the immersion of the organoid promoter cells or spheroids of tumorigenic cells, supported by a scaffold that will allow their growth in 3D. These are immersed in the culture medium, then supplemented with growth factors that can vary according to the type of tissue. The culture media that have been reported for the cultivation of organoids are Dulbecco’s modified Eagle medium (DMEM), Eagle’s minimum essential medium (EMEM), Roswell Park Memorial Institute (RPMI) medium, and Ham’s F12 culture medium (F12). This is conducted in the presence or absence of fetal bovine or equine serum and antibiotics, in addition to other supplements, such as L-glutamine, HEPES buffer, and GlutaMAX supplement; however, the critical components of the organoid media are a set of growth factors that include epidermal growth factor (EGF), fibroblast growth Factor 10 (FGF10), hepatocyte growth factor (HGF), R-spondin 1, and noggin. Figure 4a shows the general scheme of this technique for the development of organoids.
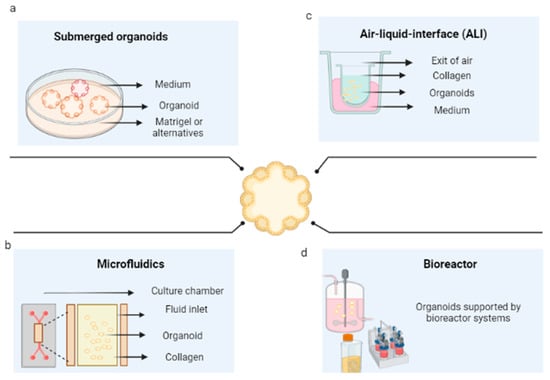
Figure 4. Organoid culture techniques. (a) Culture of organoids immersed in an extracellular matrix. (b) Organoids cultured in microfluidics. (c) Organoids cultured in an air–liquid interface (d) Organoid culture in bioreactors. Created with BioRender.com.
For example, in the development of hepatic organoids, Lugli et al. (2016) determined that the growth of these organoids was stimulated by R-spondin 1 and noggin, while in their absence, they partially differentiated into hepatocytes. For pulmonary organoids, changes in morphology were found depending on the presence of EGF, where the organoids were significantly smaller in the absence of this growth factor [137,138,139]. However, it is necessary to improve the understanding of a possible optimal cocktail of growth factors, depending on each tissue, to be closer to the sample of interest [140].
2.2. 3D Microfluidics
Three-dimensional microfluidic cultures consist of organoids generated from cells embedded within a collagen gel in the middle of a microfluidic culture device. They consist of different materials (polydimethylsiloxane, silicon, glass, polycarbonate, polymethylmethacrylate, polystyrene, cyclic olefinic polymers, and polyimide) on which straight channels or more complex structures are molded. Through these, the fluid that will pass over the microchannels is pumped [141]; in this case, supplemented culture medium (as presented in the submerged 3D cultures) flows from the channels located on both sides of the central region [142], as shown in Figure 4b. This system is particularly beneficial for its application in cancer, given the interactions that occur between the tumor microenvironment and the tumor and the possibility of regulating them [143].
Applying this strategy, Wang et al. (2013) cultured the A549 tumor cell line submerged in BME (R&D Systems, MN) as a substitute for ECM to achieve three-dimensional growth in the cell culture chamber, which was connected to syringe pumps through each of the inlets to drive the fluid flow at a rate of 0.1 µL/min of medium or drug. The experimental results showed that this is a good model for 3D growth, allowing the evaluation of protein expression [144]. Likewise, for breast cancer, organoids were generated on a chip by coculturing phenotypically normal and diseased cells and tumor nodules, finding that in this system, it was possible to recapitulate the luminal environment of the breast [145].
2.3. Air–Liquid Interface Culture
Organoid culture in an air–liquid interface (ALI) is a method that consists of seeding the set of cells derived from the tumor in a transwell dish. These cells are exposed to the culture medium at the base of the dish, which can acquire oxygen through a matrix that surrounds them and interacts with the air [146] (see Figure 4c). From this platform, it has been possible to successfully obtain organoids for colon and pancreatic cancer, among others [147,148]. For example, the normal and tumor tissues of the patients were included in a collagen gel and cultured using an ALI culture system. Additionally, renal cell carcinoma was cultured on this platform in the presence of cells of the immunological system by Esser et al. (2020), where the tissue was fragmented and cultured in the collagen-based ALI system, and organoids were generated for which it was possible to determine the correspondence with the tumor of origin by IHC staining, RNA sequencing, and drug response [149]. The main advantage of this methodology is that not only the genetic alterations of the tumor, but also the complex cellular composition and architecture of the tumor environment, can be recapitulated.
2.4. Bioreactors
Bioreactors are generally defined as devices in which biological and/or biochemical processes are developed under close supervision and strictly controlled environmental and operational conditions [150]. These restrictions allow the possibility of controlling environmental conditions, such as oxygen stress, pH, temperature, shear stress, sterility [151], aeration, and nutrient distribution, which, in turn, allow the growth of complex structures. However, they must be designed based on a comprehensive understanding of the biological and engineering aspects; that is, the operating conditions must be specified (see Figure 4d) (119). To counteract the low efficiencies in seeding and nonuniform cell distributions within the scaffolds, bioreactors are presented as an alternative, given the possibility of controlling culture conditions; with them, organoids have been generated through bioreactors.
For example, Skardal et al. (2014) developed a liver tumor organoid system in which HepG2 cells and HCT-116 metastatic colon carcinoma cells were cultured in rotating bioreactors with hyaluronic acid and gelatin microcarrier beads, which led to the initiation and growth of cell aggregates [152]. Lancaster et al. (2014) developed compared the static culture of colorectal cancer samples with a perfusion bioreactor, showing that the organoids obtained by the bioreactor maintain the architecture of the tumor tissue and the densities of the proliferating tumor cells to a significantly higher degree. In addition, static cultures emulate the characteristics of the ECM, which can contribute to the evaluation of the response to the tumor drug in a specific context of the patient [153].
2.5. In Silico Models
Tumor organoids also allow the modeling of the morphologies of experimental multicellular culture systems, serving as a basis to preselect possible experiments before performing them (129) and providing optimization for preclinical trials. In principle, they simulate cell growth and morphology of the scaffolds that would support the organoid; for example, Pang et al. (2019) designed a micro-scaffold with computer-aided design (CAD) software to find the optimal characteristics of the matrix that would support the organoid, and in this cell line, Hep G2, TMNK-1, and Swiss 3T3 were cocultured. This design was shown to be more efficient in achieving a high number of retained cells and liver functions [154]. Likewise, a prediction of the organoid model of intestinal tissue determined that these organoids cannot grow in rigid and flat substrates, and that the expansion of stem cells in an organoid depends significantly on its biomechanics [155].
Organoid culture models are being actively developed to improve the pharmacogenomic similarities between preclinical models and tumors; for example, Kong et al. (2020) integrated pharmacological data derived from in vitro tests for colorectal and bladder cancer tumor organoids. Through network-based methods and machine learning, it predicted patient responses to medications based on the interaction between the main protein networks and drugs [156]. Kather et al. (2018) designed an in silico model, computationally based on human colorectal cancer, that also includes lymphocytes, macrophages, fibrotic stroma, and necrosis, and can develop large tumors of more than 106 cells in a few minutes with standard computer hardware. This model accurately recapitulates the behavior at the cellular and tissue levels based on changes in the structure of the cell or the extracellular matrix [157].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14122709
This entry is offline, you can click here to edit this entry!
