Artificial insemination (AI) is a widely used technique in swine production. Advances in the technique have made it possible to store spermatozoa at temperatures of 15–20 °C for short periods, up to ten days. Unfortunately, it is currently associated with bacterial contamination of semen during collection and dilution. Although the temperature is reduced to induce sperm inactivity during storage, bacterial growth can still occur. Bacterial growth has been associated with deleterious effects on semen quality and shelf life, such as sperm agglutination, decreased sperm motility and viability. In addition, reproductive output after AI can also be affected by bacteriospermia.
- antibiotics
- antibiotic resistance
- bacteria contamination
- bacteriospermia
- boar semen
1. Bacterial Presence in Boar Semen
2. Semen Cold Storage and Bacterial Contamination
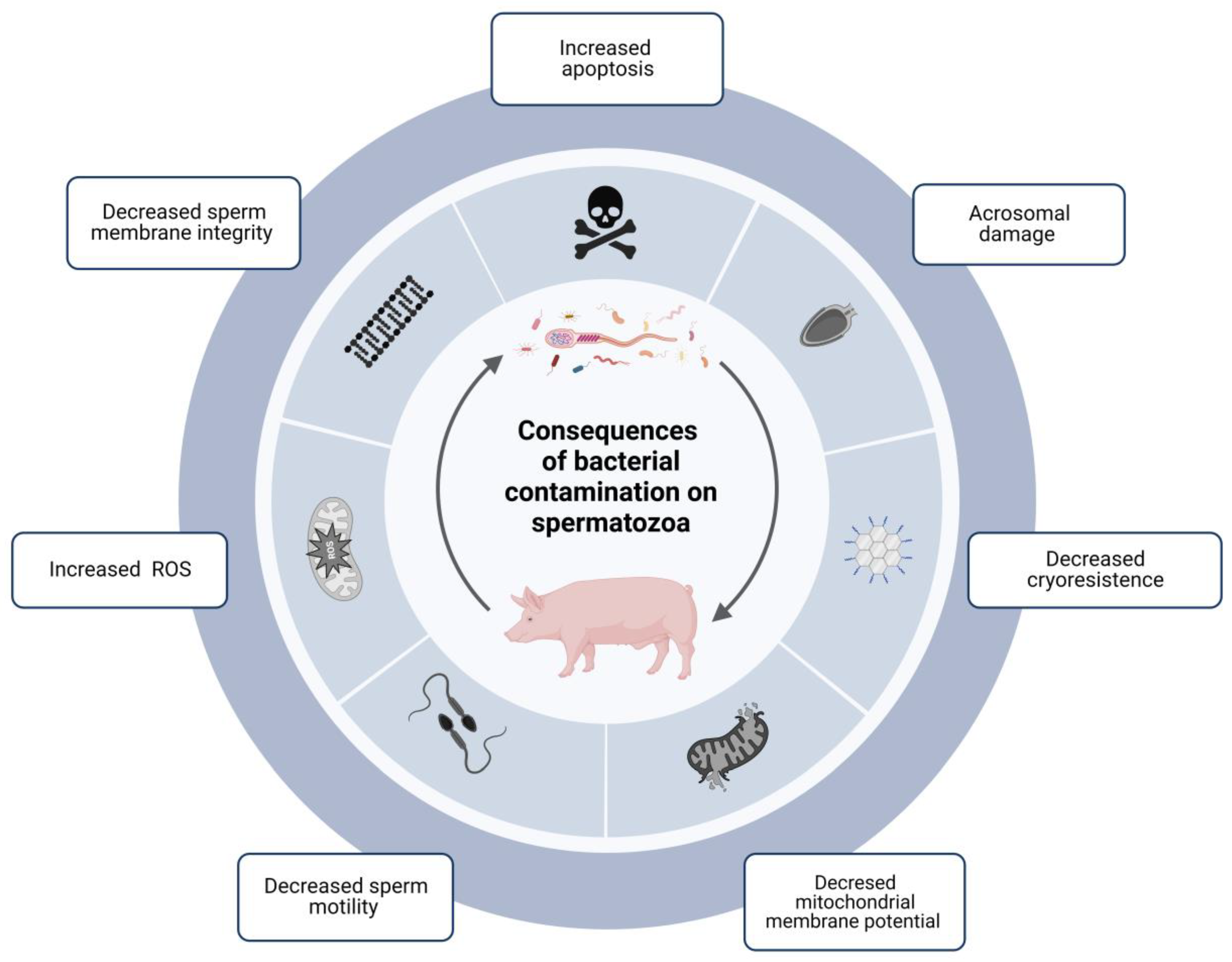
3. Consequences of Bacterial Contamination on Boar Semen
4. Antibiotic Use for Preservation of Boar Semen
This entry is adapted from the peer-reviewed paper 10.3390/antibiotics11121796
References
- Kuster, C.E.; Althouse, G.C. The Impact of Bacteriospermia on Boar Sperm Storage and Reproductive Performance. Theriogenology 2016, 85, 21–26.
- Althouse, G.C.; Lu, K.G. Bacteriospermia in Extended Porcine Semen. Theriogenology 2005, 63, 573–584.
- Althouse, G.C.; Pierdon, M.S.; Lu, K.G. Thermotemporal Dynamics of Contaminant Bacteria and Antimicrobials in Extended Porcine Semen. Theriogenology 2008, 70, 1317–1323.
- Schulze, M.; Ammon, C.; Rüdiger, K.; Jung, M.; Grobbel, M. Analysis of Hygienic Critical Control Points in Boar Semen Production. Theriogenology 2015, 83, 430–437.
- Ciornei, Ş.; Drugociu, D.; Ciornei, L.M.; Mareş, M.; Roşca, P. Total Aseptization of Boar Semen, to Increase the Biosecurity of Reproduction in Swine. Molecules 2021, 26, 6183.
- Althouse, G.C.; Kuster, C.E.; Clark, S.G.; Weisiger, R.M. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology 2000, 53, 1167–1176.
- Úbeda, J.L.; Ausejo, R.; Dahmani, Y.; Falceto, M.V.; Usan, A.; Malo, C.; Perez-Martinez, F.C. Adverse Effects of Members of the Enterobacteriaceae Family on Boar Sperm Quality. Theriogenology 2013, 80, 565–570.
- Gòdia, M.; Ramayo-Caldas, Y.; Zingaretti, L.M.; Darwich, L.; López, S.; Rodríguez-Gil, J.E.; Yeste, M.; Sánchez, A.; Clop, A. A Pilot RNA-Seq Study in 40 Pietrain Ejaculates to Characterize the Porcine Sperm Microbiome. Theriogenology 2020, 157, 525–533.
- Zhang, J.; Liu, H.; Yang, Q.; Li, P.; Wen, Y.; Han, X.; Li, B.; Jiang, H.; Li, X. Genomic Sequencing Reveals the Diversity of Seminal Bacteria and Relationships to Reproductive Potential in Boar Sperm. Front. Microbiol. 2020, 11, 1873.
- Costinar, L.; Herman, V.; Pitoiu, E.; Iancu, I.; Degi, J.; Hulea, A.; Pascu, C. Boar Semen Contamination: Identification of Gram-Negative Bacteria and Antimicrobial Resistance Profile. Animals 2022, 12, 43.
- Dalmutt, A.C.; Moreno, L.Z.; Gomes, V.T.M.; Cunha, M.P.V.; Barbosa, M.R.F.; Sato, M.I.Z.; Knöbl, T.; Pedroso, A.C.; Moreno, A.M. Characterization of Bacterial Contaminants of Boar Semen: Identification by MALDI-TOF Mass Spectrometry and Antimicrobial Susceptibility Profiling. J. Appl. Anim. Res. 2020, 48, 559–565.
- Scheinpflug, K.; Schiller, S.; Jäkel, H.; Schulze, M.; Waberski, D.; Mühldorfer, K. Relevance of Leptospira in Boar and for the Development of Alternative Antimicrobial Concepts in Boar Semen Preservation. Porc. Health Manag. 2020, 6, 31.
- Even, G.; Mottais, D.; Morien, F.; Pham, M.D.; Ostergaard, A.; Martel, S.; Merlin, S.; Audebert, C. Porcine Bacteriospermia Examined by High-Throughput Sequencing. Theriogenology 2020, 142, 268–275.
- Schulze, M.; Jung, M.; Hensel, B. Science-Based Quality Control in Boar Semen Production. Mol. Reprod. Dev. 2022. early view.
- Nitsche-Melkus, E.; Bortfeldt, R.; Jung, M.; Schulze, M. Impact of Hygiene on Bacterial Contamination in Extended Boar Semen: An Eight-Year Retrospective Study of 28 European AI Centers. Theriogenology 2020, 146, 133–139.
- Goldberg, A.M.G.; Argenti, L.E.; Faccin, J.E.; Linck, L.; Santi, M.; Bernardi, M.L.; Cardoso, M.R.I.; Wentz, I.; Bortolozzo, F.P. Risk Factors for Bacterial Contamination during Boar Semen Collection. Res. Vet. Sci. 2013, 95, 362–367.
- Stojanov, I.; Milovanović, A.; Barna, T.; Prodanov Radulović, J.; Apić, J.; Stojanović, D.; Maksimović, N. Antimicrobial Resistance as a Problem for the Quality of Boar Semen. Acta Vet. Brno. 2020, 70, 136–146.
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M.C. Storage of Boar Semen. Anim. Reprod. Sci. 2000, 62, 143–172.
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of Preserved Boar Semen for Artificial Insemination: Past, Present and Future Challenges. Theriogenology 2019, 137, 2–7.
- Yeste, M. State-of-the-Art of Boar Sperm Preservation in Liquid and Frozen State. Anim. Reprod. 2017, 14, 69–81.
- Schulze, M.; Nitsche-Melkus, E.; Jakop, U.; Jung, M.; Waberski, D. New Trends in Production Management in European Pig AI Centers. Theriogenology 2019, 137, 88–92.
- Menezes, T.d.A.; Mellagi, A.P.G.; da Silva Oliveira, G.; Bernardi, M.L.; Wentz, I.; Ulguim, R.d.R.; Bortolozzo, F.P. Antibiotic-Free Extended Boar Semen Preserved under Low Temperature Maintains Acceptable in-Vitro Sperm Quality and Reduces Bacterial Load. Theriogenology 2020, 149, 131–138.
- Balogun, K.B.; Stewart, K.R. Effects of Air Exposure and Agitation on Quality of Stored Boar Semen Samples. Reprod. Domest. Anim. 2021, 56, 1200–1208.
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Bacterial Contamination of Boar Semen and Its Relationship to Sperm Quality Preserved in Commercial Extender Containing Gentamicin Sulfate. Pol. J. Vet. Sci. 2016, 19, 451–459.
- Goldberg, A.M.G.; Cardoso, M.; Bernardi, M.L.; Wentz, I.; Bortolozzo, F.P. The Impact of Bacterial Contamination of the Ejaculate and Extender on the Quality of Swine Semen Doses. Semin. Cienc. Agrar. 2017, 38, 3095–3103.
- Bussalleu, E.; Yeste, M.; Sepúlveda, L.; Torner, E.; Pinart, E.; Bonet, S. Effects of Different Concentrations of Enterotoxigenic and Verotoxigenic E. Coli on Boar Sperm Quality. Anim. Reprod. Sci. 2011, 127, 176–182.
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Bonet, S. Effect of Pseudomonas Aeruginosa on Sperm Capacitation and Protein Phosphorylation of Boar Spermatozoa. Theriogenology 2016, 85, 1421–1431.
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Bonet, S. Effects of Different Concentrations of Pseudomonas Aeruginosa on Boar Sperm Quality. Anim. Reprod. Sci. 2014, 150, 96–106.
- Sepúlveda, L.; Bussalleu, E.; Yeste, M.; Torner, E.; Bonet, S. How Do Different Concentrations of Clostridium Perfringens Affect the Quality of Extended Boar Spermatozoa? Anim. Reprod. Sci. 2013, 140, 83–91.
- Prieto-Martínez, N.; Bussalleu, E.; Garcia-Bonavila, E.; Bonet, S.; Yeste, M. Effects of Enterobacter Cloacae on Boar Sperm Quality during Liquid Storage at 17 °C. Anim. Reprod. Sci. 2014, 148, 72–82.
- Pinart, E.; Domènech, E.; Bussalleu, E.; Yeste, M.; Bonet, S. A Comparative Study of the Effects of Escherichia Coli and Clostridium Perfringens upon Boar Semen Preserved in Liquid Storage. Anim. Reprod. Sci. 2017, 177, 65–78.
- Schulze, M.; Jakop, U.; Jung, M.; Cabezón, F. Influences on Thermo-Resistance of Boar Spermatozoa. Theriogenology 2019, 127, 15–20.
- Ďuračka, M.; Tvrda, E. The Presence of Bacterial Species in Boar Semen and Their Impact on the Sperm Quality and Oxida-Tive Balance. J. Anim. Sci. 2018, 96, 501.
- Rodriguez, A.L.; van Soom, A.; Arsenakis, I.; Maes, D. Boar Management and Semen Handling Factors Affect the Quality of Boar Extended Semen. Porc. Health Manag. 2017, 3, 15.
- Schulze, M.; Schäfer, J.; Simmet, C.; Jung, M.; Gabler, C. Detection and Characterization of Lactobacillus Spp. In the Porcine Seminal Plasma and Their Influence on Boar Semen Quality. PLoS ONE 2018, 13, e0202699.
- Bonet, S.; Delgado-Bermúdez, A.; Yeste, M.; Pinart, E. Study of Boar Sperm Interaction with Escherichia Coli and Clostridium Perfringens in Refrigerated Semen. Anim. Reprod. Sci. 2018, 197, 134–144.
- Delgado-Bermúdez, A.; Bonet, S.; Yeste, M.; Pinart, E. Long-Term Storage of Boar Seminal Doses Contaminated with Proteus Vulgaris: A Dose-Dependent Effect on Sperm Motility and Sperm-Bacteria Interaction. Anim. Reprod. Sci. 2020, 216, 106349.
- Gao, H.; Gao, Y.; Yang, C.; Dong, D.; Yang, J.; Peng, G.; Peng, J.; Wang, Y.; Pan, C.; Dong, W. Influence of Outer Membrane Vesicles of Proteus Mirabilis Isolated from Boar Semen on Sperm Function. Vet. Microbiol. 2018, 224, 34–42.
- Okazaki, T.; Mihara, T.; Fujita, Y.; Yoshida, S.; Teshima, H.; Shimada, M. Polymyxin B Neutralizes Bacteria-Released Endotoxin and Improves the Quality of Boar Sperm during Liquid Storage and Cryopreservation. Theriogenology 2010, 74, 1691–1700.
- Bryła, M.; Trzcińska, M. Quality and Fertilizing Capacity of Boar Spermatozoa during Liquid Storage in Extender Supplemented with Different Antibiotics. Anim. Reprod. Sci. 2015, 163, 157–163.
- Feng, T.Y.; Ren, F.; Fang, Q.; Dai, G.C.; Li, Y.; Li, Q.; Xi, H.M.; Li, H.; Hao, Y.Y.; Hu, J.H. Effects of Sulfanilamide on Boar Sperm Quality, Bacterial Composition, and Fertility during Liquid Storage at 17 °C. Anim. Sci. J. 2019, 90, 1161–1169.
- Luther, A.M.; Nguyen, T.Q.; Verspohl, J.; Waberski, D. Antimicrobially Active Semen Extenders Allow the Reduction of Antibiotic Use in Pig Insemination. Antibiotics 2021, 10, 1319.
- Schulze, M.; Grobbel, M.; Riesenbeck, A.; Brüning, S.; Schaefer, J.; Jung, M.; Grossfeld, R. Dose Rates of Antimicrobial Substances in Boar Semen Preservation—Time to Establish New Protocols. Reprod. Domest. Anim. 2017, 52, 397–402.
- Tvrdá, E.; Bučko, O.; Rojková, K.; Ďuračka, M.; Kunová, S.; Kováč, J.; Benko, F.; Kačániová, M. The Efficiency of Selected Extenders against Bacterial Contamination of Boar Semen in a Swine Breeding Facility in Western Slovakia. Animals 2021, 11, 3320.
- Gòdia, M.; Ramayo-Caldas, Y.; Zingaretti, L.M.; López, S.; Rodriguez-Gil, J.E.; Yeste, M.; Sánchez, A.; Clop, A. A RNA-Seq Characterization of the Porcine Sperm Microbiome. bioRxiv 2020.
