Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
The usage of peptide-based drugs to combat cancer is gaining significance in the pharmaceutical industry. The collateral damage caused to normal cells due to the use of chemotherapy, radiotherapy, etc. has given an impetus to the search for alternative methods of cancer treatment.
- ACPs (anti-cancer peptides)
- peptide drugs
- immunomodulation
1. Introduction
Rudolf Virchow first observed that persistent inflammation transcends into cancer and tumor tissues show a large infiltration of inflammatory cells. Later on, Dvorak showed that carcinogenesis and inflammation have commonalities in terms of proliferation, migration, cytokine secretion and angiogenesis. He described cancer as a “wound that does not heal” [1]. In modern times, the three main methods of cancer treatment so far have been Chemotherapy, radiotherapy and immunotherapy. Chemotherapy is the most established method of treatment that kill fast-dividing cancer cells. However, most cancer drugs have very poor cell selectivity and kill normal cells along with cancer cells indiscriminately [2,3]. Moreover, continuous use of this therapy increases the possibility of drug resistance in the body along with the chances of recurrence. Radiotherapy is the second type of cancer therapy that uses high-energy beams to kill cancer cells. X-rays are the most commonly used energy beams but, protons or other types of energy can also be used. Unfortunately, radiotherapy causes collateral damage as, despite the advancements in modern types of equipment, the radiation kills normal cells along with the targeted cancer cells. Immunotherapy is the third kind of therapy that improves the patient’s immune system to exert an anti-tumor effect. This treatment method has fewer side effects than chemotherapy and radiotherapy, and the therapeutic effects are long-lasting [4]. As a standard job, the immune system detects cancerous cells due to the presence of abnormal cell surface markers. Biopsies of patients show various immune cells in and around tumors [5]. These cells, called tumor-infiltrating lymphocytes or TILs, are a sign that the immune system is responding to the tumor. Cancer patients whose tumors contain TILs often have a lesser level of cancer severity than those whose tumors do not contain them. The interrelationship between inflammation, innate immunity and cancer is well known [6]. Persistent inflammation triggers cancer initiation that is characterized by infiltration of mononuclear immune cells (including macrophages, lymphocytes, and plasma cells), tissue destruction, fibrosis, and increased angiogenesis [7].
2. Where Anti-Cancer Peptides Stand
The discovery of peptide hormone insulin gave an impetus to peptide therapeutics. During the last two decades, peptides have grown as encouraging healing mediators in many areas such as diabetes [8], cardiovascular diseases [9] and cancer treatment [10] (Figure 1). Improvements in peptide design have increased its applications in other fields as well [11,12]. The beginning of the 21st century witnessed rapid advancements in various interdisciplinary fields like analytics, structural biology Computer-assisted drug discovery, and bioinformatics tools which have made peptide design much easier and also minimized the chances of drug failure. This has led to the opening of new areas of drug discovery where peptide synthesis, chemical modifications and the evaluation of biological activities can be done simultaneously that speed up the process of lead molecule identifications. Insights in the global market for peptide therapeutics is projected to record a value of USD 44.43 billion in 2026, progressing at a CAGR of 6.95%, over the period 2022–2026. Currently, more than 80 peptide-based drugs are present in the market for the treatment of a wide range of diseases including cancer, osteoporosis, diabetes, etc. [13]. It is estimated that up to 400–600 peptide drugs are in preclinical trials. After 2017, USFDA has already approved more than 10 peptide-based drugs. Of these, LupkynisTM and Zegalogue were recently approved in 2021, while ImcivreeTM, Victoza, LUPRON DEPOT, Zoladex, Sandostatin and Somatuline received approval in 2020 [14]. The three drugs which have touched global sales of over $1 billion are goserelin, leuprolide and octreotide [15]. Goserelin is a synthetic decapeptide analog of luteinizing hormone-releasing hormone (LHRH). It has anti-cancer activity and is used in treatments of both breast and prostate cancer [16] Leuprolide is a peptide analog of gonadotropin-releasing hormone (GnRH) which is used as a palliative treatment of prostate cancer and many other conditions [17]. Studies have shown that it also possesses an immunomodulatory effect. The investigations showed that Leuprolide Acetate (LA) administration to Experimental autoimmune encephalomyelitis (EAE) rats considerably inhibited the activation of NF-κB which is central to both inflammation and cancer progression. It was shown that treatment with LA reduced the production of TNF-α, IL-1β and other inflammatory cytokines which play an important role in both inflammation and cancer initiation [18]. The third successful peptide drug Octreotide is an analog of somatostatin. It helps to temporarily reduce the tumor size and diminish cancer development. Due to its large therapeutic abilities, octreotide has evolved as a backbone of clinical cancer therapeutics [19,20]. With the advent of bioinformatics tools, various short peptides have been identified from the naturally occurring proteins that have shown a high affinity for cancer cells. The structure-function relationships of anti-cancer peptides showed that certain biophysical parameters are present in these peptides that attract them to the cancer cells. Based on these parameters, several naturally occurring and synthetic peptides are being identified that show anti-cancerous properties.
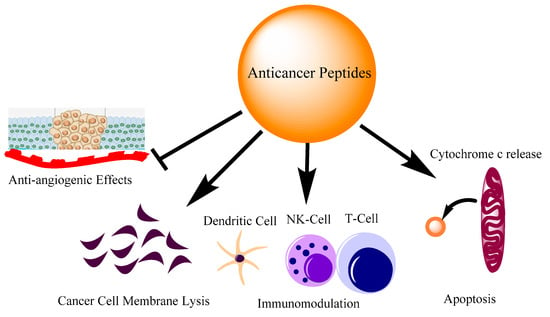
Figure 1. Different modes of action of anti-cancer peptides. The action of peptides involves inhibiting angiogenesis, direct cell membrane lysis, immune cell regulation and apoptosis by cytochrome c release from mitochondria.
3. Naturally Occurring AMPs/ACPs with Immunomodulatory Activities
The helicity is strongly correlated with antimicrobial and anti-cancer activity [21]. Naturally occurring anti-microbial peptides have shown potent anti-cancer activities in in vitro experiments in several independent studies (Table 1). Besides, naturally occurring proteins that are associated with cancer progression pathways have been used to design immunomodulatory peptides that can inhibit LPS-mediated inflammatory responses [22,23]. LL-37, Magainin-II, Melittin and other naturally occurring anti-microbial peptides have shown appreciable anti-cancer activity [24,25,26]. Additionally, they contain good anti-inflammatory activity which is an important immunomodulatory activity to tackle the dual problem of cancer and inflammation [27]. Melittin (GIGAVLKVLTTGLPALISWIKRKRQQ), a peptide first isolated from bee venom has shown to be a promising candidate for cancer therapy. It is an alpha-helical anticancer peptide that plays a key role in immunomodulation and inhibits proinflammatory agents. The anti-cancer properties exerted by melittin are very similar to anti-cancer drugs and involve cell cycle arrest, anti-proliferative activity on cancer cells and activation of caspases [28]. Besides these, melittin is also shown to induce apoptosis in cancer cells through ROS generation and the diffusion of mitochondrial membrane potential [29].
Table 1. Peptide and the amino acid sequences discussed in the Review.
| Sl No | Peptide Name | Sequence |
|---|---|---|
| 1 | LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES |
| 2 | Magainin II | GIGKFLHSAKKFGKAFVGEIMNS |
| 3 | Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ |
| 4 | P18 | KWKLFKKIPKFLHLAKKF |
| 5 | Tritrpticin | VRRFPWWWPFLRR |
| 6 | Indolicidin | ILPWKWPWWPWRR |
| 7 | PuroA | FPVTWKWWKWWKG |
| 8 | Chrysophsin-1 | FFGWLIKGAIHAGKAIHGLIHRRRH |
| 9 | Chrysophsin-2 | FFGWLIRGAIHAGKAIHGLIHRRRH |
| 10 | Temporin L | FVQWFSKFLGRIL |
| 11 | Temporin A | FLPLIGRVLSGIL |
| 12 | Bombinin H2 | LIGPVLGLVGSALGGLLKKI |
| 13 | Hepcidin | ICIFCCGCCHRSKCGMCCKT |
| 14 | KSL-W | KKVVFWVKFK |
| 15 | HB43 | FAKLLAKLAKKLL |
| 16 | LTX-315 * | KKWWKKW-DipK |
| 16 | KTH-222 | LKGQLRCI |
| 17 | K4R2-Nal2-S1 ** | KKKKRR-Nal-Nal-KKWRKWLAKK |
| 18 | PR-39 | RRRPRPPYLPRPRPPPFFPPRLPPRIPPGFPPRFPPRFP |
| 19 | L-K6 | IKKILSKIKKLLK |
| 20 | IK-13 | CIIKKIIKKIIKK |
| 21 | LK-13 | CLLKKLLKKLLKK |
| 22 | Alloferon | HGVSGHGQHGVHG |
| 23 | LactoferricinB (LfcinB) | FKCRRWQWRMKKLGAPSITCVRRAF |
| 24 | RADA16 | RADARADARADARADA |
| 25 | E3PA | AAAAGGGEEE |
| 26 | FLAK50 | FAKLLAKLAKKLL |
| 27 | VmCT1 | FLGALWNVAKSVF |
* Dip is β-diphenylalanine. ** Nal is β-naphthylalanine.
Unfortunately, melittin is a non-cell selective peptide and displays cytotoxicity to normal cells as well [30]. Therefore, to achieve its true therapeutic potential, suitable analogs of it are to be designed that show reduced cytotoxicity to normal cells but retain the anti-cancer activity. Asthana et al. have identified a leucine zipper motif in melittin which can be used as a switch to design bioactive analogs [31]. These analogs can be used for their anti-cancer and immunomodulatory activities but with reduced cytotoxicity to normal cells. Srivastava et al. showed that melittin can neutralize the lipopolysaccharide-induced proinflammatory pathways in RAW 264.7 and primary macrophage cells and the leucine zipper motif present in the peptide played an important role in its immunomodulatory activity [32]. Liu et al. created a bifunctional fusion protein melittin-MIL-2, which was a recombinant of melittin and a mutant IL-2 [33]. The melittin-MIL-2 displayed potent anti-cancer activities in comparison to Melittin and rIL-2 alone. The MIL-2 displayed anti-proliferative activity against cancer cells derived from different tissues. In the in vivo experiments, the MIL-2 was able to inhibit the tumor growth in liver, lung and ovary cancer cells. The investigators also showed that exposure of MIL-2 was able to reduce the ability of breast cancer cells to metastasize to the lungs. Another promising antimicrobial peptide that can be used for the dual role of anticancer and immunomodulatory activities is Magainin II (KWKLFKKIKFLHSAKKF). It was first isolated from the skin of Xenopus laevis frogs. Studies have shown that magainin II inhibited the cell proliferation of bladder cancer cells while did not cause any toxicity to normal fibroblast 3T3 cells [34]. Although Magainin II did not show effective anti-cancer activity on human breast cancer cells MDA-MB-231, it displays good anti-cancer activity against lung cancer cell line A549 [25]. It is not toxic to human immortalized epidermal cells under similar conditions. While Magainin II itself does not cause any immunomodulatory activity, hybrid peptides designed using cecropin A17 and Magainin II showed potent anti-cancer and anti-inflammatory activity [35,36]. A cecropin A–magainin II hybrid peptide called P18 (KWKLFKKIPKFLHLAKKF) was effective against human leukemia K562 cells [37]. Tang et al. showed that P18 induced necrosis in these cells instead of activating the apoptotic pathway. The mechanism of action of the peptide involved the diffusing of the plasma membrane potential in the cells after peptide exposure [37]. Nan et al. while studying the immunomodulatory activity of P18 showed that when mouse macrophage cell line RAW264.7 was challenged with LPS from E. coli in the absence or presence of P18, it inhibited the LPS-mediated production of pro-inflammatory mediators and cytokines viz. nitrite (NO), TNF-α and IL-1β [38]. This was a classic example of using two naturally occurring peptide sequences to design a hybrid peptide with immunomodulatory activity, which was not present in the parent peptide. The judicious substitution of key residues at either terminus of an anti-cancer peptide often improves its biological activity [39]. However, it is essential to make a conservative replacement so that the biophysical parameters like charge, hydrophobicity, etc. remain similar to the parent peptide. Along similar lines, Arias et al. designed improved analogs of tritrpticin (VRRFPWWWPFLRR) for potent anti-cancer activity against the Jurkat leukemia cell line [40]. By designing a series of analogs of tritrpticin, they found that if the arginines at both the termini are replaced with lysines or lysine-derivatives, it improves the cell-selectivity of the peptides towards Jurkat leukemia cells as opposed to normal peripheral blood mononuclear cells (PBMCs). Interestingly, arginine to lysine substitution also enhanced the biological activity in other sequentially similar peptides including indolicidin (ILPWKWPWWPWRR) and puroindoline A (PuroA FPVTWKWWKWWKG-NH2) [41]. Ghiselli et al. checked the anti-inflammatory activity of Indolicidin, a closely related peptide to tritrpticin in two rat models of polymicrobial peritonitis. The investigators used two different models to induce sepsis. One by intraperitoneal injection of LPS and the other by using cecal ligation and puncture (CLP model) of inflammation. The results showed that indolicidin treated group decreased the bacterial burden in visceral organs like peritoneum, spleen and liver and plasma levels of LPS-mediated production of TNF-α and IL-6 were also inhibited. This signifies the dual role of the peptide as both an immunomodulatory and anticancer peptide in addition to being a well-established antimicrobial peptide [42].
Anti-cancer peptides with immunomodulatory activities have also been reported from marine sources [43]. Marine fishes are rich sources of anti-cancer peptides possessing immunomodulatory activities [44]. Marine animals possess poor immune systems and live in ecological niches where they are exposed to diverse pathogens. Iijima et al. purified Chrysophsins in marine fish Chrysophsis major. There are three different forms of chrysophsins [45]. The majority of the anti-cancer and immunomodulatory studies are done on two isoforms, Chrysophsin-I (FFGWLIKGAIHAGKAIHGLIHRRRH) and Chrysophsin-II (FFGWLIRGAIHAGKAIHGLIHRRRH). Hsu et al. checked the anti-cancer activity of Chrysophsin-1. Their results showed that the peptide followed a lytic mechanism to kill cancer cells mostly through pore formation. The inhibition ratio was less for normal cell lines viz. NIH-3T3 and WS-1 [46]. Tripathi et al. identified the GXXXXG motif in Chrysophsin I and designed various proline-substituted analogs of the Chrysophsin-1 and showed that the peptides in addition to the anti-cancer activity as described by other workers also possess potent immunomodulatory activity. The authors were able to show that one of the proline-substituted analogs rescued the mice from the lethal dose of LPS [47]. Temporin L (FVQWFSKFLGRIL), another highly studied alpha-helical anti-microbial peptide has significant anti-cancer and immunomodulatory activities [48]. Swithenbank et al., while studying the activity of Temporins and Bombinin H2 (LIGPVLGLVGSALGGLLKKI) on lung cancer cell lines reported that Temporin L exposure to cancer cell lines viz. A-549 and Calu-3 caused significant cytotoxicity in a dose-dependent manner [49]. Srivastava et al. investigated the immunomodulatory activity of Temporin L. They identified a phenylalanine zipper in Temporin L that can be used to design Temporin L analogs that are less toxic to normal cells and exhibit anti-endotoxin activities [50]. Studies have shown that Temporin L directly binds to LPS and can be a therapeutic agent in septic shock [51].
Interestingly, phenylalanine heptad repeats have also been used to design synthetic peptides that contain potent anti-cancer and immunomodulatory activities. Tripathi et al. showed that if the phenylalanine residues are replaced with proline in a synthetic peptide designed on the basis of phenylalanine heptad repeats, the resultant peptide exhibit potent anti-cancer and immunomodulatory activities. The proline substituted analogs of parent peptide designed on phenylalanine heptad repeats also inhibit migration in MDA-MB-231 breast cancer cells and induce programmed cell death by activating the intrinsic pathway of apoptosis. The same peptides contained anti-endotoxin activities as they inhibit the LPS-mediated NF-kB nuclear translocation and inhibit the production of pro-inflammatory cytokines [52]. Hepcidin (ICIFCCGCCHRSKCGMCCKT) is also a good example of an anti-cancer peptide containing immunomodulatory activity [53]. Cytotoxicity data of hepcidin on myeloma cells indicate that it causes plasma membrane damage and DNA fragmentation in these cancer cells to exhibit its anti-cancerous activities [54]. An independent study about the immunomodulatory activity of hepcidin showed that it can up-regulate the expression of both pro- and anti-inflammatory cytokines like TNF-α, IL-1β, and IL-10 in teleost leukocytes. [53]. The mRNA expression was also found to be high in the organs like the spleen and head kidney. LL-37(LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) is probably the most widely studied Cathelicidin. Originally investigated for its antimicrobial activities, LL-37 soon was reported to inhibit a wide range of cancers and is very context-specific [55]. The role of LL-37 in colon cancer seems to be most interesting. A differential expression of LL-37 has been observed in normal colon and cancer colon mucosa. It had been reported that LL-37 gets downregulated as colon cancer progresses [56]. This has led to the idea of using LL-37 as a colon cancer biomarker [57]. It can also induce apoptosis by upregulating the levels of Bax/Bak and downregulating the BCL-2 levels [58]. LL-37 has also been shown to increase the PUMA (p53 upregulated modulator of apoptosis) expression which is a modulator of apoptosis in colon cancer cells [24]. Besides this, LL-37 also increases the nuclear translocation of apoptosis-inducing-factor (AIF) and endonuclease G (EndoG) in colon cancer cells to induce apoptosis [56]. FK-16, a derivate of full-length LL-37 containing the same amino acid residues from 17 to 32 followed a similar mechanism as the parental LL-37 to cause apoptosis by increasing the nuclear levels of apoptosis-inducing-factor (AIF) and endonuclease G (EndoG) in colon cancer cells in a caspase-independent manner [59]. LL-37 also has a very potent immunomodulatory activity on different types of immune cells. It is shown to have antisepsis properties and has been proven to neutralize the inflammatory responses activated by bacterial components like LPS and LTA. Culturing the bone marrow-derived macrophages with LPS with or without LL-37 showed that the LL-37 was able to almost completely cancel out the LPS-mediated TNF-α and brought it to an almost basal level [60].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14122686
This entry is offline, you can click here to edit this entry!
